Bactericidal/permeability-increasing protein promotes complement activation for neutrophil-mediated phagocytosis on bacterial surface
- PMID: 11529944
- PMCID: PMC1783264
- DOI: 10.1046/j.1365-2567.2001.01263.x
Bactericidal/permeability-increasing protein promotes complement activation for neutrophil-mediated phagocytosis on bacterial surface
Abstract
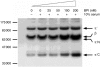
The neutrophil bactericidal/permeability-increasing protein (BPI) has both bactericidal and lipopolysaccharide-neutralizing activities. The present study suggests that BPI also plays an important role in phagocytosis of Escherichia coli by neutrophils through promotion of complement activation on the bacterial surface. Flow cytometric analysis indicated that fluorescein-labelled E. coli treated with BPI were phagocytosed in the presence of serum at two- to five-fold higher levels than phagocytosis of the bacteria without the treatment. In contrast, phagocytosis of the fluoresceined bacteria with or without treatment by BPI did not occur at all in the absence of serum. The phagocytosis stimulated by BPI and serum was dose-dependent. The effect of BPI on phagocytosis in the presence of serum was not observed on Gram-positive bacteria (Staphylococcus aureus). Interestingly, the complement C3b/iC3b fragments were deposited onto the bacterial surface also as a function of the BPI concentration under conditions similar to those for phagocytosis. Furthermore, the BPI-promoted phagocytosis was blocked completely by anti-C3 F(ab')(2) and partially by anti-complement receptor (CR) type 1 and/or anti-CR type 3. These findings suggest that BPI accelerates complement activation to opsonize bacteria with complement-derived fragments, leading to stimulation of phagocytosis by neutrophils via CR(s).
Figures




Similar articles
-
Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein.Pediatrics. 1999 Dec;104(6):1327-33. doi: 10.1542/peds.104.6.1327. Pediatrics. 1999. PMID: 10585984
-
An opsonic function of the neutrophil bactericidal/permeability-increasing protein depends on both its N- and C-terminal domains.Proc Natl Acad Sci U S A. 1997 Sep 30;94(20):10973-8. doi: 10.1073/pnas.94.20.10973. Proc Natl Acad Sci U S A. 1997. PMID: 9380744 Free PMC article.
-
Sensitivity of K1-encapsulated Escherichia coli to killing by the bactericidal/permeability-increasing protein of rabbit and human neutrophils.Infect Immun. 1982 Dec;38(3):1149-53. doi: 10.1128/iai.38.3.1149-1153.1982. Infect Immun. 1982. PMID: 6759406 Free PMC article.
-
The bactericidal/permeability-increasing protein (BPI), a potent element in host-defense against gram-negative bacteria and lipopolysaccharide.Immunobiology. 1993 Apr;187(3-5):417-29. doi: 10.1016/S0171-2985(11)80354-2. Immunobiology. 1993. PMID: 8330906 Review.
-
Therapeutic potential of the bactericidal/permeability-increasing protein.Expert Opin Investig Drugs. 2002 Feb;11(2):159-67. doi: 10.1517/13543784.11.2.159. Expert Opin Investig Drugs. 2002. PMID: 11829710 Review.
Cited by
-
Bactericidal/Permeability-Increasing Protein Preeminently Mediates Clearance of Pseudomonas aeruginosa In Vivo via CD18-Dependent Phagocytosis.Front Immunol. 2021 Apr 26;12:659523. doi: 10.3389/fimmu.2021.659523. eCollection 2021. Front Immunol. 2021. PMID: 33981306 Free PMC article.
-
Comparison of capillary and venous blood in the analysis of concentration and function of leucocyte sub-populations.Eur J Appl Physiol. 2016 Aug;116(8):1583-93. doi: 10.1007/s00421-016-3413-z. Epub 2016 Jun 15. Eur J Appl Physiol. 2016. PMID: 27306382
-
The Impact of BPI Expression on Escherichia coli F18 Infection in Porcine Kidney Cells.Animals (Basel). 2020 Nov 15;10(11):2118. doi: 10.3390/ani10112118. Animals (Basel). 2020. PMID: 33203175 Free PMC article.
-
Identification and Functional Analysis of ToBPI1/LBP and ToBPI2/LBP in Anti-Bacterial Infection of Trachinotus ovatus.Genes (Basel). 2023 Mar 29;14(4):826. doi: 10.3390/genes14040826. Genes (Basel). 2023. PMID: 37107584 Free PMC article.
-
Neutrophil bactericidal function is defective in patients with recurrent urinary tract infections.Urol Res. 2003 Oct;31(5):329-34. doi: 10.1007/s00240-003-0344-z. Epub 2003 Jul 31. Urol Res. 2003. PMID: 14574538
References
-
- Raetz CRH. Biochemistry of endotoxin. Annu Rev Biochem. 1990;59:129–70. - PubMed
-
- Ulevitch RJ. Recognition of bacterial endotoxins by receptor-dependent mechanisms. Adv Immunol. 1993;53:267–89. - PubMed
-
- Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

