MP20, the second most abundant lens membrane protein and member of the tetraspanin superfamily, joins the list of ligands of galectin-3
- PMID: 11532191
- PMCID: PMC48140
- DOI: 10.1186/1471-2121-2-17
MP20, the second most abundant lens membrane protein and member of the tetraspanin superfamily, joins the list of ligands of galectin-3
Abstract
Background: Although MP20 is the second most highly expressed membrane protein in the lens its function remains an enigma. Putative functions for MP20 have recently been inferred from its assignment to the tetraspanin superfamily of integral membrane proteins. Members of this family have been shown to be involved in cellular proliferation, differentiation, migration, and adhesion. In this study, we show that MP20 associates with galectin-3, a known adhesion modulator.
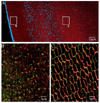
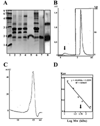
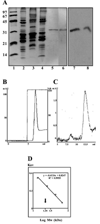
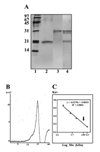
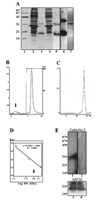
Results: MP20 and galectin-3 co-localized in selected areas of the lens fiber cell plasma membrane. Individually, these proteins purified with apparent molecular masses of 60 kDa and 22 kDa, respectively. A 104 kDa complex was formed in vitro upon mixing the purified proteins. A 102 kDa complex of MP20 and galectin-3 could also be isolated from detergent-solubilized native fiber cell membranes. Binding between MP20 and galectin-3 was disrupted by lactose suggesting the lectin site was involved in the interaction.
Conclusions: MP20 adds to a growing list of ligands of galectin-3 and appears to be the first representative of the tetraspanin superfamily identified to possess this specificity.
Figures
Similar articles
-
Polymorphic assemblies and crystalline arrays of lens tetraspanin MP20.J Mol Biol. 2008 Feb 15;376(2):380-92. doi: 10.1016/j.jmb.2007.09.001. Epub 2007 Sep 8. J Mol Biol. 2008. PMID: 18166196
-
Phosphorylation and glycosylation of bovine lens MP20.Invest Ophthalmol Vis Sci. 2005 Feb;46(2):627-35. doi: 10.1167/iovs.04-0894. Invest Ophthalmol Vis Sci. 2005. PMID: 15671292
-
Galectin-3 is associated with the plasma membrane of lens fiber cells.Invest Ophthalmol Vis Sci. 2000 Jan;41(1):199-203. Invest Ophthalmol Vis Sci. 2000. PMID: 10634621 Free PMC article.
-
Galectin-3 as a multifunctional protein.Cell Mol Biol Lett. 2004;9(2):305-28. Cell Mol Biol Lett. 2004. PMID: 15213811 Review.
-
Galectin-3 and metastasis.Glycoconj J. 2002;19(7-9):543-9. doi: 10.1023/B:GLYC.0000014084.01324.15. Glycoconj J. 2002. PMID: 14758078 Review.
Cited by
-
A novel mutation of LIM2 causes autosomal dominant membranous cataract in a Chinese family.Int J Ophthalmol. 2020 Oct 18;13(10):1512-1520. doi: 10.18240/ijo.2020.10.02. eCollection 2020. Int J Ophthalmol. 2020. PMID: 33078099 Free PMC article.
-
Analysis of binding of mannosides in relation to Langerin (CD207) in Langerhans cells of normal and transformed epithelia.Histochem J. 2002 May;34(5):247-53. doi: 10.1023/a:1021793530802. Histochem J. 2002. PMID: 12588002
-
A missense mutation in LIM2 causes autosomal recessive congenital cataract.Mol Vis. 2008 Jun 23;14:1204-8. Mol Vis. 2008. PMID: 18596884 Free PMC article.
-
Elongated axial length and myopia-related fundus changes associated with the Arg130Cys mutation in the LIM2 gene in four Chinese families with congenital cataracts.Ann Transl Med. 2021 Feb;9(3):235. doi: 10.21037/atm-20-4275. Ann Transl Med. 2021. PMID: 33708862 Free PMC article.
-
Novel and known variants in GJA3 and LIM2 in congenital cataract families from North India.BMC Genomics. 2024 Jan 4;25(1):31. doi: 10.1186/s12864-023-09880-7. BMC Genomics. 2024. PMID: 38178039 Free PMC article.
References
-
- Maecker HT, Todd SC, Levy S. The tetraspanin superfamily – molecular facilitators. FASEB J. 1997;11:428–442. - PubMed
-
- Serru V, Naour FLe, Billard M, Azorsa DO, Lanza F, Boucheix C, Rubenstein E. Selective tetraspan-integrin complexes (CD81/alpha 4 beta 1, CD151/alpha 3 beta 1, CD151/alpha6 beta 1) under conditions disrupting tetraspan interactions. Biochem J. 1999;340:103–111. doi: 10.1042/0264-6021:3400103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

