Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment
- PMID: 11532928
- PMCID: PMC125600
- DOI: 10.1093/emboj/20.17.4639
Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment
Abstract
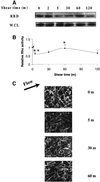
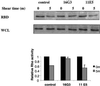
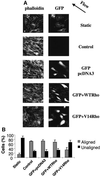
Fluid shear stress is a critical determinant of vascular remodeling and atherogenesis. Both integrins and the small GTPase Rho are implicated in endothelial cell responses to shear but the mechanisms are poorly understood. We now show that shear stress rapidly stimulates conformational activation of integrin alpha(v)beta3 in bovine aortic endothelial cells, followed by an increase in its binding to extracellular cell matrix (ECM) proteins. The shear-induced new integrin binding to ECM induces a transient inactivation of Rho similar to that seen when suspended cells are plated on ECM proteins. This transient inhibition is necessary for cytoskeletal alignment in the direction of flow. The results therefore define the role of integrins and Rho in a pathway leading to endothelial cell adaptation to flow.
Figures




References
-
- Arthur W.T., Petch,L.A. and Burridge,K. (2000) Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol., 10, 719–722. - PubMed
-
- Avnur Z. and Geiger,B. (1981) The removal of extracellular fibronectin from areas of cell–substrate contact. Cell, 25, 121–132. - PubMed
-
- Barbee K.A., Davies,P.F. and Lal,R. (1994) Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ. Res., 74, 163–171. - PubMed
-
- Bazzoni G. and Hemler,M.E. (1998) Are changes in integrin affinity and conformation overemphasized? Trends Biochem. Sci., 23, 30–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

