The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation
- PMID: 11532957
- PMCID: PMC125268
- DOI: 10.1093/emboj/20.17.4935
The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation
Abstract
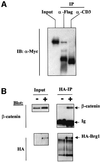
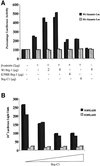
Wnt-induced formation of nuclear Tcf-beta-catenin complexes promotes transcriptional activation of target genes involved in cell fate decisions. Inappropriate expression of Tcf target genes resulting from mutational activation of this pathway is also implicated in tumorigenesis. The C-terminus of beta-catenin is indispensable for the transactivation function, which probably reflects the presence of binding sites for essential transcriptional coactivators such as p300/CBP. However, the precise mechanism of transactivation remains unclear. Here we demonstrate an interaction between beta-catenin and Brg-1, a component of mammalian SWI/SNF and Rsc chromatin-remodelling complexes. A functional consequence of reintroduction of Brg-1 into Brg-1-deficient cells is enhanced activity of a Tcf-responsive reporter gene. Consistent with this, stable expression of inactive forms of Brg-1 in colon carcinoma cell lines specifically inhibits expression of endogenous Tcf target genes. In addition, we observe genetic interactions between the Brg-1 and beta-catenin homologues in flies. We conclude that beta-catenin recruits Brg-1 to Tcf target gene promoters, facilitating chromatin remodelling as a prerequisite for transcriptional activation.
Figures




References
-
- Adams C.C. and Workman,J.L. (1993) Nucleosome displacement in transcription. Cell, 72, 305–308. - PubMed
-
- Behrens J., von Kries,J.P., Kuhl,M., Bruhn,L., Wedlich,D., Grosschedl,R. and Birchmeier,W. (1996) Functional interaction of β-catenin with the transcription factor LEF-1. Nature, 382, 638–642. - PubMed
-
- Bienz M. and Clevers,H. (2000) Linking colorectal cancer to Wnt signaling. Cell, 103, 311–320. - PubMed
-
- Blomquist P., Li,Q. and Wrange,O. (1996) The affinity of nuclear factor 1 for its DNA site is drastically reduced by nucleosome organization irrespective of its rotational or translational position. J. Biol. Chem., 271, 153–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

