Antigenically distinct conformations of CXCR4
- PMID: 11533159
- PMCID: PMC114464
- DOI: 10.1128/JVI.75.19.8957-8967.2001
Antigenically distinct conformations of CXCR4
Abstract
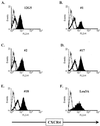
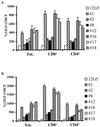
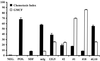
The major human immunodeficiency virus type 1 (HIV-1) coreceptors are the chemokine receptors CCR5 and CXCR4. The patterns of expression of the major coreceptors and their use by HIV-1 strains largely explain viral tropism at the level of entry. However, while virus infection is dependent upon the presence of CD4 and an appropriate coreceptor, it can be influenced by a number of factors, including receptor concentration, affinity between envelope gp120 and receptors, and potentially receptor conformation. Indeed, seven-transmembrane domain receptors, such as CCR5, can exhibit conformational heterogeneity, although the significance for virus infection is uncertain. Using a panel of monoclonal antibodies (MAbs) to CXCR4, we found that CXCR4 on both primary and transformed T cells as well as on primary B cells exhibited considerable conformational heterogeneity. The conformational heterogeneity of CXCR4 explains the cell-type-dependent ability of CXCR4 antibodies to block chemotaxis to stromal cell-derived factor 1 alpha and to inhibit HIV-1 infection. In addition, the MAb most commonly used to study CXCR4 expression, 12G5, recognizes only a subpopulation of CXCR4 molecules on all primary cell types analyzed. As a result, CXCR4 concentrations on these important cell types have been underestimated to date. Finally, while the factors responsible for altering CXCR4 conformation are not known, we found that they do not involve CXCR4 glycosylation, sulfation of the N-terminal domain of CXCR4, or pertussis toxin-sensitive G-protein coupling. The fact that this important HIV-1 coreceptor exists in multiple conformations could have implications for viral entry and for the development of receptor antagonists.
Figures





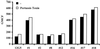
References
-
- Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. - PubMed
-
- Bleul C C, Farazan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. - PubMed
-
- Brelot A, Heveker N, Montes M, Alizon M. Identification of residues of CXCR4 critical for human immunodeficiency virus coreceptor and chemokine receptor activities. J Biol Chem. 2000;275:23736–23744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

