Tyrosine phosphorylation of bovine herpesvirus 1 tegument protein VP22 correlates with the incorporation of VP22 into virions
- PMID: 11533164
- PMCID: PMC114469
- DOI: 10.1128/JVI.75.19.9010-9017.2001
Tyrosine phosphorylation of bovine herpesvirus 1 tegument protein VP22 correlates with the incorporation of VP22 into virions
Abstract
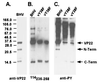
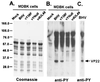
Tyrosine phosphorylation has been shown to play a role in the replication of several herpesviruses. In this report, we demonstrate that bovine herpesvirus 1 infection triggered tyrosine phosphorylation of proteins with molecular masses similar to those of phosphorylated viral structural proteins. One of the tyrosine-phosphorylated viral structural proteins was the tegument protein VP22. A tyrosine 38-to-phenylalanine mutation totally abolished the phosphorylation of VP22 in transfected cells. However, construction of a VP22 tyrosine 38-to-phenylalanine mutant virus demonstrated that VP22 was still phosphorylated but that the phosphorylation site may change to the C terminus rather than be in the N terminus as in wild-type VP22. In addition, the loss of VP22 tyrosine phosphorylation correlated with reduced incorporation of VP22 compared to that of envelope glycoprotein D in the mutant viruses but not with the amount of VP22 produced during virus infection. Our data suggest that tyrosine phosphorylation of VP22 plays a role in virion assembly.
Figures




Similar articles
-
Bovine Herpesvirus 1 UL49.5 Interacts with gM and VP22 To Ensure Virus Cell-to-Cell Spread and Virion Incorporation: Novel Role for VP22 in gM-Independent UL49.5 Virion Incorporation.J Virol. 2018 Jun 13;92(13):e00240-18. doi: 10.1128/JVI.00240-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29669828 Free PMC article.
-
Deletion of the herpes simplex virus VP22-encoding gene (UL49) alters the expression, localization, and virion incorporation of ICP0.J Virol. 2005 Aug;79(15):9735-45. doi: 10.1128/JVI.79.15.9735-9745.2005. J Virol. 2005. PMID: 16014935 Free PMC article.
-
Phosphorylation of Bovine Herpesvirus 1 VP8 Plays a Role in Viral DNA Encapsidation and Is Essential for Its Cytoplasmic Localization and Optimal Virion Incorporation.J Virol. 2016 Apr 14;90(9):4427-4440. doi: 10.1128/JVI.00219-16. Print 2016 May. J Virol. 2016. PMID: 26889039 Free PMC article.
-
Functional roles of the tegument proteins of herpes simplex virus type 1.Virus Res. 2009 Nov;145(2):173-86. doi: 10.1016/j.virusres.2009.07.007. Epub 2009 Jul 15. Virus Res. 2009. PMID: 19615419 Review.
-
Role of human cytomegalovirus tegument proteins in virion assembly.Viruses. 2014 Feb 6;6(2):582-605. doi: 10.3390/v6020582. Viruses. 2014. PMID: 24509811 Free PMC article. Review.
Cited by
-
Regulation and function of phosphorylation on VP8, the major tegument protein of bovine herpesvirus 1.J Virol. 2015 Apr;89(8):4598-611. doi: 10.1128/JVI.03180-14. Epub 2015 Feb 11. J Virol. 2015. PMID: 25673708 Free PMC article.
-
The role of the cytoskeleton in the life cycle of viruses and intracellular bacteria: tracks, motors, and polymerization machines.Curr Drug Targets Infect Disord. 2002 Sep;2(3):247-64. doi: 10.2174/1568005023342407. Curr Drug Targets Infect Disord. 2002. PMID: 12462128 Free PMC article.
-
Physical interaction between envelope glycoproteins E and M of pseudorabies virus and the major tegument protein UL49.J Virol. 2002 Aug;76(16):8208-17. doi: 10.1128/jvi.76.16.8208-8217.2002. J Virol. 2002. PMID: 12134026 Free PMC article.
-
Actin is a component of the compensation mechanism in pseudorabies virus virions lacking the major tegument protein VP22.J Virol. 2005 Jul;79(13):8614-9. doi: 10.1128/JVI.79.13.8614-8619.2005. J Virol. 2005. PMID: 15956602 Free PMC article.
-
Protein kinase Cdc7 supports viral replication by phosphorylating Avibirnavirus VP3 protein.J Virol. 2023 Nov 30;97(11):e0112523. doi: 10.1128/jvi.01125-23. Epub 2023 Oct 30. J Virol. 2023. PMID: 37902398 Free PMC article.
References
-
- Baird A, Florkiewicz R Z, Maher P A, Kaner R J, Hajjar D P. Mediation of virion penetration into vascular cells by association of basic fibroblast growth factor with herpes simplex virus type 1. Nature. 1990;348:344–346. - PubMed
-
- Blaho J A, Mitchell C, Roizman B. An amino acid sequence shared by the herpes simplex virus 1 alpha regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J Biol Chem. 1994;269:17401–17410. - PubMed
-
- Coulter L J, Moss H W, Lang J, McGeoch D J. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J Gen Virol. 1993;74:387–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

