Tyrosine phosphorylation of bovine herpesvirus 1 tegument protein VP22 correlates with the incorporation of VP22 into virions
- PMID: 11533164
- PMCID: PMC114469
- DOI: 10.1128/JVI.75.19.9010-9017.2001
Tyrosine phosphorylation of bovine herpesvirus 1 tegument protein VP22 correlates with the incorporation of VP22 into virions
Abstract
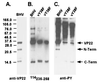
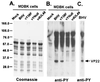
Tyrosine phosphorylation has been shown to play a role in the replication of several herpesviruses. In this report, we demonstrate that bovine herpesvirus 1 infection triggered tyrosine phosphorylation of proteins with molecular masses similar to those of phosphorylated viral structural proteins. One of the tyrosine-phosphorylated viral structural proteins was the tegument protein VP22. A tyrosine 38-to-phenylalanine mutation totally abolished the phosphorylation of VP22 in transfected cells. However, construction of a VP22 tyrosine 38-to-phenylalanine mutant virus demonstrated that VP22 was still phosphorylated but that the phosphorylation site may change to the C terminus rather than be in the N terminus as in wild-type VP22. In addition, the loss of VP22 tyrosine phosphorylation correlated with reduced incorporation of VP22 compared to that of envelope glycoprotein D in the mutant viruses but not with the amount of VP22 produced during virus infection. Our data suggest that tyrosine phosphorylation of VP22 plays a role in virion assembly.
Figures




References
-
- Baird A, Florkiewicz R Z, Maher P A, Kaner R J, Hajjar D P. Mediation of virion penetration into vascular cells by association of basic fibroblast growth factor with herpes simplex virus type 1. Nature. 1990;348:344–346. - PubMed
-
- Blaho J A, Mitchell C, Roizman B. An amino acid sequence shared by the herpes simplex virus 1 alpha regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J Biol Chem. 1994;269:17401–17410. - PubMed
-
- Coulter L J, Moss H W, Lang J, McGeoch D J. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J Gen Virol. 1993;74:387–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

