The conserved serine 177 in the delta antigen of hepatitis delta virus is one putative phosphorylation site and is required for efficient viral RNA replication
- PMID: 11533172
- PMCID: PMC114477
- DOI: 10.1128/JVI.75.19.9087-9095.2001
The conserved serine 177 in the delta antigen of hepatitis delta virus is one putative phosphorylation site and is required for efficient viral RNA replication
Abstract
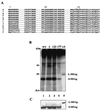
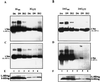
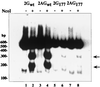
Hepatitis delta virus (HDV) small delta antigen (S-HDAg) plays a critical role in virus replication. We previously demonstrated that the S-HDAg phosphorylation occurs on both serine and threonine residues. However, their biological significance and the exact phosphorylation sites of S-HDAg are still unknown. In this study, phosphorylated S-HDAg was detected only in the intracellular compartment, not in viral particles. In addition, the number of phosphorylated isoforms of S-HDAg significantly increased with the extent of viral replication in transfection system. Site-directed mutagenesis showed that alanine replacement of serine 177, which is conserved among all the known HDV strains, resulted in reduced phosphorylation of S-HDAg, while the mutation of the other two conserved serine residues (2 and 123) had little effect. The S177A mutant dramatically decreased its capability in assisting HDV RNA replication, with a preferential and profound impairment of the antigenomic RNA replication. Furthermore, the viral RNA editing, a step relying upon antigenomic RNA replication, was also abolished by this mutation. These results suggested that phosphorylation of S-HDAg, with serine 177 as a presumable site, plays a critical role in viral RNA replication, especially in augmenting the replication of antigenomic RNA.
Figures




Similar articles
-
Casein kinase II and protein kinase C modulate hepatitis delta virus RNA replication but not empty viral particle assembly.J Virol. 1996 Sep;70(9):6190-8. doi: 10.1128/JVI.70.9.6190-6198.1996. J Virol. 1996. PMID: 8709245 Free PMC article.
-
Assembly of hepatitis delta virus particles: package of multimeric hepatitis delta virus genomic RNA and role of phosphorylation.Virology. 1998 Sep 15;249(1):12-20. doi: 10.1006/viro.1998.9310. Virology. 1998. PMID: 9740772
-
Recombinant hepatitis delta antigen from E. coli promotes hepatitis delta virus RNA replication only from the genomic strand but not the antigenomic strand.Virology. 2000 Dec 20;278(2):578-86. doi: 10.1006/viro.2000.0680. Virology. 2000. PMID: 11118380
-
The molecular biology of hepatitis delta virus.Annu Rev Biochem. 1995;64:259-86. doi: 10.1146/annurev.bi.64.070195.001355. Annu Rev Biochem. 1995. PMID: 7574482 Review.
-
Hepatitis delta virus. Genetics and pathogenesis.Clin Lab Med. 1996 Jun;16(2):451-64. Clin Lab Med. 1996. PMID: 8792082 Review.
Cited by
-
Hepatitis delta: virological and clinical aspects.Virol J. 2017 Sep 13;14(1):177. doi: 10.1186/s12985-017-0845-y. Virol J. 2017. PMID: 28903779 Free PMC article. Review.
-
Multiple Regions Drive Hepatitis Delta Virus Proliferation and Are Therapeutic Targets.Front Microbiol. 2022 Apr 6;13:838382. doi: 10.3389/fmicb.2022.838382. eCollection 2022. Front Microbiol. 2022. PMID: 35464929 Free PMC article. Review.
-
Characterization of hepatitis B and delta coinfection in Israel.BMC Infect Dis. 2018 Feb 27;18(1):97. doi: 10.1186/s12879-018-3008-x. BMC Infect Dis. 2018. PMID: 29486716 Free PMC article.
-
A novel toolbox for the in vitro assay of hepatitis D virus infection.Sci Rep. 2017 Jan 12;7:40199. doi: 10.1038/srep40199. Sci Rep. 2017. PMID: 28079152 Free PMC article.
-
RNA replication without RNA-dependent RNA polymerase: surprises from hepatitis delta virus.J Virol. 2005 Jul;79(13):7951-8. doi: 10.1128/JVI.79.13.7951-7958.2005. J Virol. 2005. PMID: 15956541 Free PMC article. Review. No abstract available.
References
-
- Cartier C, Sivard P, Tranchat C, Decimo D, Desgranges C, Boyer V. Identification of three major phosphorylation sites within HIV-1 capsid. Role of phosphorylation during the early steps of infection. J Biol Chem. 1999;274:19434–19440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

