Receptor binding transforms the surface subunit of the mammalian C-type retrovirus envelope protein from an inhibitor to an activator of fusion
- PMID: 11533173
- PMCID: PMC114478
- DOI: 10.1128/JVI.75.19.9096-9105.2001
Receptor binding transforms the surface subunit of the mammalian C-type retrovirus envelope protein from an inhibitor to an activator of fusion
Abstract
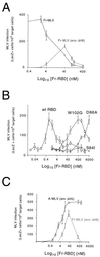
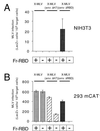
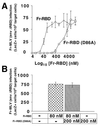
The envelope protein (Env) of murine leukemia viruses (MLVs) is composed of a surface subunit (SU) and a transmembrane subunit (TM), which mediates membrane fusion, resulting in infection. SU contains a discrete N-terminal receptor binding domain (RBD) that is connected to the remainder of Env by a short, proline-rich segment. Previous studies suggest that after receptor binding, the RBD interacts directly with the remainder of Env to trigger fusion (A. L. Barnett, R. A. Davey, and J. M. Cunningham, Proc. Natl. Acad. Sci. USA 98:4113-4118, 2001). To investigate the role of the RBD in activating fusion, we compared infection by several MLVs that are defective unless rescued in trans by the addition of soluble RBD to the culture medium. Infection by MLV lacking a critical histidine residue near the N terminus of the viral RBD is dependent on the expression of receptors for both the RBD in the viral Env and the soluble RBD supplied in trans. However, infection by MLVs in which the RBD has been deleted or replaced by the ligand erythropoietin are dependent only on expression of the receptor for the soluble RBD. We were able to expand the host range of xenotropic MLV to nonpermissive murine fibroblasts only if the RBD was deleted from the xenotropic viral envelope and the soluble RBD from ecotropic Friend MLV was supplied to the culture medium. These findings indicate that receptor binding transforms the RBD from an inhibitor to an activator of the viral fusion mechanism and that viruses lacking the critical histidine residue at the N terminus of the RBD are impaired at the activation step.
Figures



References
-
- Albritton L M, Tseng L, Scadden D, Cunningham J M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. - PubMed
-
- Anderson M M, Lauring A S, Burns C C, Overbaugh J. Identification of a cellular cofactor required for infection by feline leukemia virus. Science. 2000;287:1828–1830. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

