Induction of p53-independent apoptosis by simian virus 40 small t antigen
- PMID: 11533178
- PMCID: PMC114483
- DOI: 10.1128/JVI.75.19.9142-9155.2001
Induction of p53-independent apoptosis by simian virus 40 small t antigen
Abstract
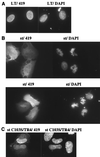
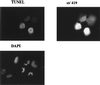
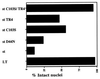
Simian virus 40 small t antigen (st) is required for optimal transformation and replication properties of the virus. We find that in certain cell types, such as the human osteosarcoma cell line U2OS, st is capable of inducing apoptosis, as evidenced by a fragmented nuclear morphology and positive terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling staining of transfected cells. The cell death can be p53 independent, since it also occurs in p53-deficient H1299 cells. Genetic analysis indicates that two specific mutants affect apoptosis induction. One of these (C103S) has been frequently used as a PP2A binding mutant. The second mutant (TR4) lacks the final four amino acids of st, which have been reported to be unimportant for PP2A binding in vitro. However, TR4 unexpectedly fails to bind PP2A in vivo. Furthermore, a long-term colony assay reveals a potent colony inhibition upon st expression, and the behavior of st mutants in this assay reflects the relative frequency of nuclear fragmentation observed in transfections using the same mutants. Notably, either Bcl-2 coexpression or broad caspase inhibitor treatment could restore normal nuclear morphology. Finally, fluorescence-activated cell sorting analysis suggests a correlation between the ability of st to modulate cell cycle progression and apoptosis. Taken together, these observations underscore that st does not always promote proliferation but may, depending on conditions and cell type, effect a cell death response.
Figures
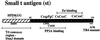




Similar articles
-
The c-Myc-interacting adaptor protein Bin1 activates a caspase-independent cell death program.Oncogene. 2000 Sep 28;19(41):4669-84. doi: 10.1038/sj.onc.1203681. Oncogene. 2000. PMID: 11032017
-
Inhibition of SV40 large T antigen induced apoptosis by small T antigen.Oncogene. 1999 Sep 30;18(40):5598-603. doi: 10.1038/sj.onc.1202942. Oncogene. 1999. PMID: 10523837
-
The role of the SV40 ST antigen in cell growth promotion and transformation.Semin Cancer Biol. 2001 Feb;11(1):5-13. doi: 10.1006/scbi.2000.0341. Semin Cancer Biol. 2001. PMID: 11243894 Review.
-
Activation of the tumor-specific death effector apoptin and its kinase by an N-terminal determinant of simian virus 40 large T antigen.J Virol. 2004 Sep;78(18):9965-76. doi: 10.1128/JVI.78.18.9965-9976.2004. J Virol. 2004. PMID: 15331730 Free PMC article.
-
[Apoptosis and human viral infections].Ann Biol Clin (Paris). 1996;54(5):189-97. Ann Biol Clin (Paris). 1996. PMID: 8869358 Review. French.
Cited by
-
Akt inhibitors as an HIV-1 infected macrophage-specific anti-viral therapy.Retrovirology. 2008 Jan 31;5:11. doi: 10.1186/1742-4690-5-11. Retrovirology. 2008. PMID: 18237430 Free PMC article.
-
KSHV LANA acetylation-selective acidic domain reader sequence mediates virus persistence.Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22443-22451. doi: 10.1073/pnas.2004809117. Epub 2020 Aug 20. Proc Natl Acad Sci U S A. 2020. PMID: 32820070 Free PMC article.
-
Simian virus 40 large T antigen and two independent T-antigen segments sensitize cells to apoptosis following genotoxic damage.J Virol. 2002 Aug;76(16):8420-32. doi: 10.1128/jvi.76.16.8420-8432.2002. J Virol. 2002. PMID: 12134045 Free PMC article.
-
G9a stimulates CRC growth by inducing p53 Lys373 dimethylation-dependent activation of Plk1.Theranostics. 2018 Apr 15;8(10):2884-2895. doi: 10.7150/thno.23824. eCollection 2018. Theranostics. 2018. PMID: 29774081 Free PMC article.
-
Protein phosphatase 2A regulates life and death decisions via Akt in a context-dependent manner.Proc Natl Acad Sci U S A. 2007 Nov 27;104(48):19011-6. doi: 10.1073/pnas.0706696104. Epub 2007 Nov 15. Proc Natl Acad Sci U S A. 2007. PMID: 18006659 Free PMC article.
References
-
- Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams W V, Green D R, Weiner D B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat Med. 1997;3:1117–1123. - PubMed
-
- Banks L, Edmonds C, Vousden K H. Ability of the HPV16 E7 protein to bind RB and induce DNA synthesis is not sufficient for efficient transforming activity in NIH3T3 cells. Oncogene. 1990;5:1383–1389. - PubMed
-
- Bergqvist A, Magnusson G. Apoptosis of Spodoptera frugiperda cells induced by okadaic acid is abrogated by baculovirus infection. Exp Cell Res. 1994;215:223–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

