Comparative pathogenesis of tissue culture-adapted and wild-type Cowden porcine enteric calicivirus (PEC) in gnotobiotic pigs and induction of diarrhea by intravenous inoculation of wild-type PEC
- PMID: 11533186
- PMCID: PMC114491
- DOI: 10.1128/JVI.75.19.9239-9251.2001
Comparative pathogenesis of tissue culture-adapted and wild-type Cowden porcine enteric calicivirus (PEC) in gnotobiotic pigs and induction of diarrhea by intravenous inoculation of wild-type PEC
Abstract
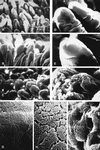
Porcine enteric calicivirus (PEC/Cowden) causes diarrhea in pigs, grows in cell culture, and is morphologically and genetically similar to the Sapporo-like human caliciviruses. Genetic analysis revealed that the tissue culture-adapted (TC) Cowden PEC has one distant and three clustered amino acid substitutions in the capsid region and 2 amino acid changes in the RNA polymerase region compared to wild-type (WT) PEC (M. Guo, K.-O. Chang, M. E. Hardy, Q. Zhang, A. V. Parwani, and L. J. Saif, J. Virol. 73:9625-9631, 1999). In this study, the TC PEC, passaged in a porcine kidney cell line, and the WT PEC, passaged in gnotobiotic (Gn) pigs, were used to orally inoculate 13 4- to 6-day-old Gn pigs. No diarrhea developed in the TC-PEC-exposed pigs, whereas moderate diarrhea developed in the WT-PEC orally inoculated pigs, persisting for 2 to 5 days. Fecal virus shedding persisting for at least 7 days was detected by both reverse transcription (RT)-PCR and antigen-enzyme-linked immunosorbent assay (antigen-ELISA) in both TC-PEC and WT-PEC orally inoculated pigs but not in mock-inoculated pigs. The PEC particles were detected by immunoelectron microscopy (IEM) in intestinal contents from all the WT-PEC-inoculated pigs, but not from the TC-PEC-inoculated pigs. Mild (duodenum and jejunum) or no (ileum) villous atrophy was observed in histologic sections of the small intestines of TC-PEC-inoculated pigs, whereas WT PEC caused mild to severe (duodenum and jejunum) villous atrophy and fusion. Scanning electron microscopy confirmed mild shortening and blunting of villi in the duodenum and jejunum of the TC-PEC-inoculated pigs, in contrast to moderate to severe villous shortening and blunting in the duodenum and jejunum of WT-PEC-inoculated pigs. Higher numbers of PEC antigen-positive villous enterocytes were detected by immunofluorescent (IF) staining in the proximal small intestine of the WT-PEC-inoculated pigs, in contrast to low numbers of PEC antigen-positive enterocytes in only one of four TC-PEC-inoculated pigs. No PEC antigen-positive cells were observed in the colon or extraintestinal tissues of all inoculated pigs or in the small intestine of one mock-inoculated pig. Thus, the TC PEC was at least partially attenuated (no diarrhea, mild lesions) after serial passage in cell culture. In further experiments, three 4- to 6-day-old Gn pigs were intravenously (i.v.) inoculated with WT PEC, and all pigs developed diarrhea and villous atrophy in the small intestines resembling that observed in the orally inoculated pigs. Fecal viral shedding persisting for 8 days was detected by both RT-PCR and antigen-ELISA, and PEC was detected by IEM in feces or intestinal contents. The PEC RNA and antigens (at low titers) were detected in acute-phase sera from all the WT-PEC i.v.-inoculated pigs and also from seven of nine of the WT-PEC orally inoculated pigs. Oral or i.v. inoculation of four additional pigs with the PEC-positive acute-phase sera induced diarrhea, small intestinal lesions, PEC shedding in feces, and seroconversion to PEC, confirming the occurrence of viremia during PEC infection, with infectious PEC present in acute-phase sera. No diarrhea, histopathologic changes, or IF staining in the small intestine or fecal or serum detection of PEC was evident in two pigs i.v. mock-inoculated or a pig inoculated i.v. with inactivated WT PEC. To our knowledge, this is the first report of an attenuated enteric calicivirus, the induction of diarrhea, and intestinal lesions in Gn pigs caused by i.v. inoculation of WT PEC and the presence of viremia following PEC infection.
Figures

References
-
- Agus S G, Dolin R, Wyatt R G, Tousimis A J, Northrup R S. Acute infectious nonbacterial gastroenteritis: intestinal histopathology. Ann Intern Med. 1973;79:18–25. - PubMed
-
- Blacklow N R, Dolin R, Fedson D S, Dupont H, Northrup R S, Hornick R B, Chanock R M. Acute infectious nonbacterial gastroenteritis: etiology and pathogenesis. Ann Intern Med. 1972;76:993–1008. - PubMed
-
- Bridger J C. Small viruses associated with gastroenteritis in animals. In: Saif L J, Theil K W, editors. Viral diarrheas of man and animals. Boca Raton, Fla: CRC Press; 1990. pp. 161–182.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

