Cross-linking of the fingers subdomain of human immunodeficiency virus type 1 reverse transcriptase to template-primer
- PMID: 11533206
- PMCID: PMC114511
- DOI: 10.1128/JVI.75.19.9435-9445.2001
Cross-linking of the fingers subdomain of human immunodeficiency virus type 1 reverse transcriptase to template-primer
Abstract
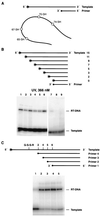
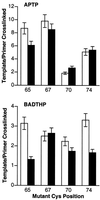
Cross-linking experiments were performed with human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) mutants with unique cysteine residues at several positions (positions 65, 67, 70, and 74) in the fingers subdomain of the p66 subunit. Two approaches were used--photoaffinity cross-linking and disulfide chemical cross-linking (using an oligonucleotide that contained an N(2)-modified dG with a reactive thiol group). In the former case, cross-linking can occur to any nucleotide in either DNA strand, and in the latter case, a specific cross-link is produced between the template and the enzyme. Neither the introduction of the unique cysteine residues into the fingers nor the modification of these residues with photocross-linking reagents caused a significant decrease in the enzymatic activities of RT. We were able to use this model system to investigate interactions between specific points on the fingers domain of RT and double-stranded DNA (dsDNA). Photoaffinity cross-linking of the template to the modified RTs with Cys residues in positions 65, 67, 70, and 74 of the fingers domain of the p66 subunit was relatively efficient. Azide-modified Cys residues produced 10 to 25% cross-linking, whereas diazirine modified residues produced 5 to 8% cross-linking. Disulfide cross-linking yields were up to 90%. All of the modified RTs preferentially photocross-linked to the 5' extended template strand of the dsDNA template-primer substrate. The preferred sites of interactions were on the extended template, 5 to 7 bases beyond the polymerase active site. HIV-1 RT is quite flexible. There are conformational changes associated with substrate binding. Cross-linking was used to detect intramolecular movements associated with binding of the incoming deoxynucleoside triphosphate (dNTP). Binding an incoming dNTP at the polymerase active site decreases the efficiency of cross-linking, but causes only modest changes in the preferred positions of cross-linking. This suggests that the interactions between the fingers of p66 and the extended template involve the "open" configuration of the enzyme with the fingers away from the active site rather than the closed configuration with the fingers in direct contact with the incoming dNTP. This experimental approach can be used to measure distances between any site on the surface of the protein and an interacting molecule.
Figures



Similar articles
-
Nonnucleoside inhibitor binding affects the interactions of the fingers subdomain of human immunodeficiency virus type 1 reverse transcriptase with DNA.J Virol. 2004 Apr;78(7):3387-97. doi: 10.1128/jvi.78.7.3387-3397.2004. J Virol. 2004. PMID: 15016861 Free PMC article.
-
Protein-nucleic acid interactions and DNA conformation in a complex of human immunodeficiency virus type 1 reverse transcriptase with a double-stranded DNA template-primer.Biopolymers. 1997;44(2):125-38. doi: 10.1002/(SICI)1097-0282(1997)44:2<125::AID-BIP2>3.0.CO;2-X. Biopolymers. 1997. PMID: 9354757 Review.
-
Crystal structures of an N-terminal fragment from Moloney murine leukemia virus reverse transcriptase complexed with nucleic acid: functional implications for template-primer binding to the fingers domain.J Mol Biol. 2000 Feb 18;296(2):613-32. doi: 10.1006/jmbi.1999.3477. J Mol Biol. 2000. PMID: 10669612
-
Structure and functional implications of the polymerase active site region in a complex of HIV-1 RT with a double-stranded DNA template-primer and an antibody Fab fragment at 2.8 A resolution.J Mol Biol. 1998 Dec 11;284(4):1095-111. doi: 10.1006/jmbi.1998.2208. J Mol Biol. 1998. PMID: 9837729
-
Molecular mechanisms of gene regulation studied by site-directed spin labeling.Methods. 2003 Feb;29(2):188-95. doi: 10.1016/s1046-2023(02)00309-2. Methods. 2003. PMID: 12606224 Review.
Cited by
-
Impact of template overhang-binding region of HIV-1 RT on the binding and orientation of the duplex region of the template-primer.Mol Cell Biochem. 2010 May;338(1-2):19-33. doi: 10.1007/s11010-009-0316-x. Epub 2009 Nov 17. Mol Cell Biochem. 2010. PMID: 19921401
-
Structures of HIV-1 reverse transcriptase with pre- and post-translocation AZTMP-terminated DNA.EMBO J. 2002 Dec 2;21(23):6614-24. doi: 10.1093/emboj/cdf637. EMBO J. 2002. PMID: 12456667 Free PMC article.
-
Nonnucleoside inhibitor binding affects the interactions of the fingers subdomain of human immunodeficiency virus type 1 reverse transcriptase with DNA.J Virol. 2004 Apr;78(7):3387-97. doi: 10.1128/jvi.78.7.3387-3397.2004. J Virol. 2004. PMID: 15016861 Free PMC article.
-
Localization of ASV integrase-DNA contacts by site-directed crosslinking and their structural analysis.PLoS One. 2011;6(12):e27751. doi: 10.1371/journal.pone.0027751. Epub 2011 Dec 1. PLoS One. 2011. PMID: 22145019 Free PMC article.
-
SplitTester: software to identify domains responsible for functional divergence in protein family.BMC Bioinformatics. 2005 Jun 1;6:137. doi: 10.1186/1471-2105-6-137. BMC Bioinformatics. 2005. PMID: 15929795 Free PMC article.
References
-
- Allerson C R, Verdine G L. Synthesis and biochemical evaluation of RNA containing an intrahelical disulfide crosslink. Chem Biol. 1995;2:667–675. - PubMed
-
- Benn S, Rutledge R, Folks T, Gold J, Baker L, McCormick J, Feorino P, Piot P, Quinn T, Martin M. Genomic heterogeneity of AIDS retroviral isolates from North America and Zaire. Science. 1985;230:949–951. - PubMed
-
- Boyer P L, Tantillo C, Jacobo-Molina A, Nanni R G, Ding J, Arnold E, Hughes S H. Sensitivity of wild-type human immunodeficiency virus type 1 reverse transcriptase to dideoxynucleotides depends on template length: the sensitivity of drug-resistant mutants does not. Proc Natl Acad Sci USA. 1994;91:4882–4886. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

