The latency-associated nuclear antigen encoded by Kaposi's sarcoma-associated herpesvirus activates two major essential Epstein-Barr virus latent promoters
- PMID: 11533207
- PMCID: PMC114512
- DOI: 10.1128/JVI.75.19.9446-9457.2001
The latency-associated nuclear antigen encoded by Kaposi's sarcoma-associated herpesvirus activates two major essential Epstein-Barr virus latent promoters
Abstract
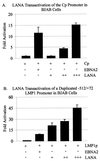
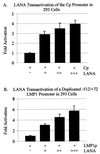
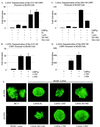
The latency-associated nuclear antigen (LANA) encoded by the Kaposi's sarcoma-associated herpesvirus (KSHV) is expressed in the majority of KSHV-infected cells and in cells coinfected with Epstein-Barr virus (EBV). In coinfected body cavity-based lymphomas (BCBLs), EBV latent membrane protein 1 (LMP1), which is essential for B-lymphocyte transformation, is expressed. EBNA2 upregulates the expression of LMP1 and other cellular genes through specific interactions with cellular transcription factors tethering EBNA2 to its responsive promoters. In coinfected BCBL cells, EBNA2 is not detected but LANA, which is constitutively expressed, contains motifs suggestive of potential transcriptional activity. Additionally, recent studies have shown that LANA is capable of activating cellular promoters. Therefore, we investigated whether LANA can affect transcription from two major EBV latent promoters. In this study, we demonstrated that LANA can efficiently transactivate both the LMP1 and C promoters in the human B-cell line BJAB as well as in the human embryonic kidney 293 cell line. Moreover, we demonstrated that specific domains of LANA containing the putative leucine zipper and the glutamic acid-rich region are highly effective in upregulating these viral promoters, while the amino-terminal region (435 amino acids) exhibited little or no transactivation activity in our assays. We also specifically tested truncations of the LMP1 promoter element and showed that the -204 to +40 region had increased levels of activation compared with a larger region, -512 to +40, which contains two recombination signal-binding protein J kappa binding sites. The smaller, -204 to +40 promoter region contains specific binding sites for the Ets family transcription factor PU.1, transcription activating factor/cyclic AMP response element, and Sp1, all of which are known to function as activators of transcription. Our data therefore suggest a potential role for LANA in regulation of the major EBV latent promoters in KSHV- and EBV-coinfected cells. Furthermore, LANA may be able to activate transcription of viral and cellular promoters in the absence of EBNA2, potentially through association with transcription factors bound to their cognate sequences within the -204 to +40 region. This regulation of viral gene expression is critical for persistence of these DNA tumor viruses and most likely involved in mediating the oncogenic process in these coinfected cells.
Figures



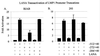


Similar articles
-
Distinct patterns of viral antigen expression in Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus coinfected body-cavity-based lymphoma cell lines: potential switches in latent gene expression due to coinfection.Virology. 1999 Sep 15;262(1):18-30. doi: 10.1006/viro.1999.9876. Virology. 1999. PMID: 10489337
-
Domains of the Epstein-Barr virus nuclear antigen 2 (EBNA2) involved in the transactivation of the latent membrane protein 1 and the EBNA Cp promoters.J Gen Virol. 1995 Nov;76 ( Pt 11):2669-78. doi: 10.1099/0022-1317-76-11-2669. J Gen Virol. 1995. PMID: 7595374
-
Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen Inhibits Major Histocompatibility Complex Class II Expression by Disrupting Enhanceosome Assembly through Binding with the Regulatory Factor X Complex.J Virol. 2015 May;89(10):5536-56. doi: 10.1128/JVI.03713-14. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740990 Free PMC article.
-
Lytic cycle switches of oncogenic human gammaherpesviruses.Adv Cancer Res. 2007;97:81-109. doi: 10.1016/S0065-230X(06)97004-3. Adv Cancer Res. 2007. PMID: 17419942 Review.
-
Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen: Replicating and Shielding Viral DNA during Viral Persistence.J Virol. 2017 Jun 26;91(14):e01083-16. doi: 10.1128/JVI.01083-16. Print 2017 Jul 15. J Virol. 2017. PMID: 28446671 Free PMC article. Review.
Cited by
-
Intracellular activated Notch1 is critical for proliferation of Kaposi's sarcoma-associated herpesvirus-associated B-lymphoma cell lines in vitro.J Virol. 2006 Jul;80(13):6411-9. doi: 10.1128/JVI.00239-06. J Virol. 2006. PMID: 16775329 Free PMC article.
-
Identification of a novel cellular transcriptional repressor interacting with the latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus.J Virol. 2003 Sep;77(18):9758-68. doi: 10.1128/jvi.77.18.9758-9768.2003. J Virol. 2003. PMID: 12941884 Free PMC article.
-
Identification of white spot syndrome virus latency-related genes in specific-pathogen-free shrimps by use of a microarray.J Virol. 2003 Sep;77(18):10162-7. doi: 10.1128/jvi.77.18.10162-10167.2003. J Virol. 2003. PMID: 12941929 Free PMC article.
-
Distinct p53, p53:LANA, and LANA complexes in Kaposi's Sarcoma--associated Herpesvirus Lymphomas.J Virol. 2010 Apr;84(8):3898-908. doi: 10.1128/JVI.01321-09. Epub 2010 Feb 3. J Virol. 2010. PMID: 20130056 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus-encoded LANA can interact with the nuclear mitotic apparatus protein to regulate genome maintenance and segregation.J Virol. 2008 Jul;82(13):6734-46. doi: 10.1128/JVI.00342-08. Epub 2008 Apr 16. J Virol. 2008. PMID: 18417561 Free PMC article.
References
-
- Ballestas M E, Chatis P A, Kaye K M. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284:641–644. - PubMed
-
- Bonnet M, Guinebretiere J M, Kremmer E, Grunewald V, Benhamou E, Contesso G, Joab I. Detection of Epstein-Barr virus in invasive breast cancers. J Natl Cancer Inst. 1999;91:1376–1381. - PubMed
-
- Burger R, Neipel F, Fleckenstein B, Savino R, Ciliberto G, Kalden J R, Gramatzki M. Human herpesvirus type 8 interleukin-6 homologue is functionally active on human myeloma cells. Blood. 1998;91:1858–1863. - PubMed
-
- Callahan J, Pai S, Cotter M, Robertson E S. Distinct patterns of viral antigen expression in Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus coinfected body-cavity-based lymphoma cell lines: potential switches in latent gene expression due to coinfection. Virology. 1999;262:18–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

