Varicella-zoster Virus gB and gE coexpression, but not gB or gE alone, leads to abundant fusion and syncytium formation equivalent to those from gH and gL coexpression
- PMID: 11533210
- PMCID: PMC114515
- DOI: 10.1128/JVI.75.19.9483-9492.2001
Varicella-zoster Virus gB and gE coexpression, but not gB or gE alone, leads to abundant fusion and syncytium formation equivalent to those from gH and gL coexpression
Abstract
Varicella-zoster virus (VZV) is distinguished from herpes simplex virus type 1 (HSV-1) by the fact that cell-to-cell fusion and syncytium formation require only gH and gL within a transient-expression system. In the HSV system, four glycoproteins, namely, gH, gL, gB, and gD, are required to induce a similar fusogenic event. VZV lacks a gD homologous protein. In this report, the role of VZV gB as a fusogen was investigated and compared to the gH-gL complex. First of all, the VZV gH-gL experiment was repeated under a different set of conditions; namely, gH and gL were cloned into the same vaccinia virus (VV) genome. Surprisingly, the new expression system demonstrated that a recombinant VV-gH+gL construct was even more fusogenic than seen in the prior experiment with two individual expression plasmids containing gH and gL (K. M. Duus and C. Grose, J. Virol. 70:8961-8971, 1996). Recombinant VV expressing VZV gB by itself, however, effected the formation of only small syncytia. When VZV gE and gB genes were cloned into one recombinant VV genome and another fusion assay was performed, extensive syncytium formation was observed. The degree of fusion with VZV gE-gB coexpression was comparable to that observed with VZV gH-gL: in both cases, >80% of the cells in a monolayer were fused. Thus, these studies established that VZV gE-gB coexpression greatly enhanced the fusogenic properties of gB. Control experiments documented that the fusion assay required a balance between the fusogenic potential of the VZV glycoproteins and the fusion-inhibitory effect of the VV infection itself.
Figures


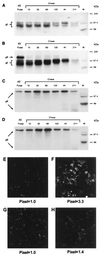



Similar articles
-
Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus.Rev Med Virol. 2003 Jul-Aug;13(4):207-22. doi: 10.1002/rmv.377. Rev Med Virol. 2003. PMID: 12820183 Review.
-
Immune response to vaccinia virus recombinants expressing glycoproteins gE, gB, gH, and gL of Varicella-zoster virus.Virology. 2001 Feb 15;280(2):211-20. doi: 10.1006/viro.2000.0754. Virology. 2001. PMID: 11162835
-
Multiple regulatory effects of varicella-zoster virus (VZV) gL on trafficking patterns and fusogenic properties of VZV gH.J Virol. 1996 Dec;70(12):8961-71. doi: 10.1128/JVI.70.12.8961-8971.1996. J Virol. 1996. PMID: 8971025 Free PMC article.
-
Cell surface expression and fusion by the varicella-zoster virus gH:gL glycoprotein complex: analysis by laser scanning confocal microscopy.Virology. 1995 Jul 10;210(2):429-40. doi: 10.1006/viro.1995.1359. Virology. 1995. PMID: 7618278
-
Varicella-zoster virus: molecular controls of cell fusion-dependent pathogenesis.Biochem Soc Trans. 2020 Dec 18;48(6):2415-2435. doi: 10.1042/BST20190511. Biochem Soc Trans. 2020. PMID: 33259590 Free PMC article. Review.
Cited by
-
A functional YNKI motif in the short cytoplasmic tail of varicella-zoster virus glycoprotein gH mediates clathrin-dependent and antibody-independent endocytosis.J Virol. 2003 Apr;77(7):4191-204. doi: 10.1128/jvi.77.7.4191-4204.2003. J Virol. 2003. PMID: 12634377 Free PMC article.
-
Characterization of the varicella-zoster virus ORF50 gene, which encodes glycoprotein M.J Virol. 2010 Apr;84(7):3488-502. doi: 10.1128/JVI.01838-09. Epub 2010 Jan 27. J Virol. 2010. PMID: 20106918 Free PMC article.
-
The transmembrane domain and cytoplasmic tail of herpes simplex virus type 1 glycoprotein H play a role in membrane fusion.J Virol. 2002 Nov;76(21):10708-16. doi: 10.1128/jvi.76.21.10708-10716.2002. J Virol. 2002. PMID: 12368313 Free PMC article.
-
Cholesterol dependence of varicella-zoster virion entry into target cells.J Virol. 2007 Jul;81(14):7548-58. doi: 10.1128/JVI.00486-07. Epub 2007 May 9. J Virol. 2007. PMID: 17494071 Free PMC article.
-
A novel recombinant ORF7-siRNA delivered by flexible nano-liposomes inhibits varicella zoster virus infection.Cell Biosci. 2023 Sep 12;13(1):167. doi: 10.1186/s13578-023-01108-1. Cell Biosci. 2023. PMID: 37700336 Free PMC article.
References
-
- Baker K A, Dutch R E, Lamb R A, Jardetzky T S. Structural basis for paramyxovirus-mediated membrane fusion. Mol Cell. 1999;3:309–319. - PubMed
-
- Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. - PubMed
-
- Bold S, Ohlin M, Garten W, Radsak K. Structural domains involved in human cytomegalovirus glycoprotein B-mediated cell-cell fusion. J Gen Virol. 1996;77:2297–2302. - PubMed
-
- Boyle D B, Coupar B E. A dominant selectable marker for the construction of recombinant poxviruses. Gene. 1988;65:123–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

