Characterization of a porcine lung epithelial cell line suitable for influenza virus studies
- PMID: 11533214
- PMCID: PMC114519
- DOI: 10.1128/JVI.75.19.9517-9525.2001
Characterization of a porcine lung epithelial cell line suitable for influenza virus studies
Abstract
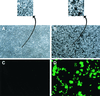
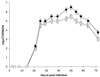
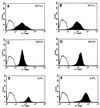
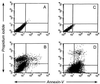
We established a porcine lung epithelial cell line designated St. Jude porcine lung cells (SJPL) and demonstrated that all tested influenza A and B viruses replicated in this cell line. The infectivity titers of most viruses in SJPL cells were comparable to or better than those in MDCK cells. The propagation of influenza viruses from clinical samples in SJPL cells did not lead to antigenic changes in the hemagglutinin molecule. The numbers of both Sia2-3Gal and Sia2-6Gal receptors on SJPL cells were greater than those on MDCK cells. Influenza virus infection of SJPL cells did not lead to apoptosis, as did infection of MDCK cells. No porcine endogenous retrovirus was detected in SJPL cells, and in contrast to MDCK cells, SJPL cells did not cause tumors in nude mice.
Figures



References
-
- Armstrong J A, Porterfield J S, Madrid A T D. C-type virus particles in pig kidney cell lines. J Gen Virol. 1971;10:195–198. - PubMed
-
- Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. - PubMed
-
- Carroll S M, Paulson J C. Differential infection of receptor-modified host cells by receptor-specific influenza viruses. Virus Res. 1985;3:165–179. - PubMed
-
- Connor R J, Kawaoka Y, Webster R G, Paulson J C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. - PubMed
-
- Duvall E, Wyllie A H. Death and the cell. Immunol Today. 1986;7:115–119. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

