Overexpression of and RNA interference with the CCAAT enhancer-binding protein on long-term facilitation of Aplysia sensory to motor synapses
- PMID: 11533225
- PMCID: PMC311377
- DOI: 10.1101/lm.40201
Overexpression of and RNA interference with the CCAAT enhancer-binding protein on long-term facilitation of Aplysia sensory to motor synapses
Abstract
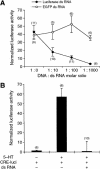
In the marine mollusk Aplysia, the CCAAT/enhancer-binding protein, ApC/EBP, serves as an immediate early gene in the consolidation of long-term facilitation in the synaptic connection between the sensory and motor neurons of the gill-withdrawal reflex. To further examine the role of ApC/EBP as a molecular switch of a stable form of long-term memory, we cloned the full-length coding regions of two alternatively spliced forms, the short and long form of ApC/EBP. Overexpression of each isoform by DNA microinjection resulted in a l6-fold increase in the expression of the coinjected luciferase reporter gene driven by an ERE promoter. In addition, when we overexpressed ApC/EBP in Aplysia sensory neurons, we found that the application of a single pulse of 5-HT that normally induced only short-term facilitation now induced long-term facilitation. Conversely, when we attempted to block the synthesis of native ApC/EBP by microinjecting double-strand RNA or antisense RNA, we blocked long-term facilitation in a sequence-specific manner. These data support the idea that ApC/EBP is both necessary and sufficient to consolidate short-term memory into long-term memory. Furthermore, our results suggest that this double-strand RNA interference provides a powerful tool in the study of the genes functioning in learning and memory in Aplysia by specifically inhibiting both the constitutive and induced expression of the genes.
Figures

Similar articles
-
Regulation of ApC/EBP mRNA by the Aplysia AU-rich element-binding protein, ApELAV, and its effects on 5-hydroxytryptamine-induced long-term facilitation.J Neurochem. 2006 Jul;98(2):420-9. doi: 10.1111/j.1471-4159.2006.03887.x. J Neurochem. 2006. PMID: 16805836
-
A nucleolar protein ApLLP induces ApC/EBP expression required for long-term synaptic facilitation in aplysia neurons.Neuron. 2006 Mar 2;49(5):707-18. doi: 10.1016/j.neuron.2006.01.035. Neuron. 2006. PMID: 16504946
-
Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation.Nature. 1990 Jun 21;345(6277):718-21. doi: 10.1038/345718a0. Nature. 1990. PMID: 2141668
-
Transcriptional regulation of long-term memory in the marine snail Aplysia.Mol Brain. 2008 Jun 17;1:3. doi: 10.1186/1756-6606-1-3. Mol Brain. 2008. PMID: 18803855 Free PMC article. Review.
-
Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture.J Neurobiol. 1994 Jun;25(6):666-93. doi: 10.1002/neu.480250608. J Neurobiol. 1994. PMID: 8071666 Review.
Cited by
-
CCAAT enhancer binding protein δ plays an essential role in memory consolidation and reconsolidation.J Neurosci. 2013 Feb 20;33(8):3646-58. doi: 10.1523/JNEUROSCI.1635-12.2013. J Neurosci. 2013. PMID: 23426691 Free PMC article.
-
The regulation of transcription in memory consolidation.Cold Spring Harb Perspect Biol. 2014 Dec 4;7(1):a021741. doi: 10.1101/cshperspect.a021741. Cold Spring Harb Perspect Biol. 2014. PMID: 25475090 Free PMC article. Review.
-
Regulation of neuronal excitability by interaction of fragile X mental retardation protein with slack potassium channels.J Neurosci. 2012 Oct 31;32(44):15318-27. doi: 10.1523/JNEUROSCI.2162-12.2012. J Neurosci. 2012. PMID: 23115170 Free PMC article.
-
Postsynaptic effects of Aplysia cysteine-rich neurotrophic factor in the induction of activity-dependent long-term facilitation in Aplysia californica.Learn Mem. 2020 Mar 16;27(4):124-129. doi: 10.1101/lm.051011.119. Print 2020 Apr. Learn Mem. 2020. PMID: 32179654 Free PMC article.
-
Learning-related synaptic growth mediated by internalization of Aplysia cell adhesion molecule is controlled by membrane phosphatidylinositol 4,5-bisphosphate synthetic pathway.J Neurosci. 2012 Nov 14;32(46):16296-305. doi: 10.1523/JNEUROSCI.1872-12.2012. J Neurosci. 2012. PMID: 23152613 Free PMC article.
References
-
- Alberini CM, Ghiradi M, Metz R, Kandel ER. ApC/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplyisa. Cell. 1994;76:1099–1114. - PubMed
-
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1983;5:397–426. - PubMed
-
- Bartsch D, Casadio A, Karl CA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. - PubMed
-
- Bartsch D, Ghirardi M, Casadio A, Giustetto M, Karl KA, Zhu H, Kandel ER. Enhancement of memory-related long-term facilitation by ApAF, a novel transcription factor that acts downstream from both CREB1 and CREB2. Cell. 2000;103:1–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
