Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability
- PMID: 11533235
- PMCID: PMC99793
- DOI: 10.1128/MCB.21.9.6461-6469.2001
Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability
Abstract
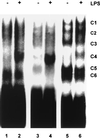
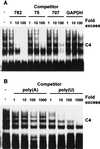
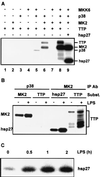
Signal transduction pathways regulate gene expression in part by modulating the stability of specific mRNAs. For example, the mitogen-activated protein kinase (MAPK) p38 pathway mediates stabilization of tumor necrosis factor alpha (TNF-alpha) mRNA in myeloid cells stimulated with bacterial lipopolysaccharide (LPS). The zinc finger protein tristetraprolin (TTP) is expressed in response to LPS and regulates the stability of TNF-alpha mRNA. We show that stimulation of RAW264.7 mouse macrophages with LPS induces the binding of TTP to the TNF-alpha 3' untranslated region. The p38 pathway is required for the induction of TNF-alpha RNA-binding activity and for the expression of TTP protein and mRNA. Following stimulation with LPS, TTP is expressed in multiple, differentially phosphorylated forms. We present evidence that phosphorylation of TTP is mediated by the p38-regulated kinase MAPKAPK2 (MAPK-activated protein kinase 2). Our findings demonstrate a direct link between a specific signal transduction pathway and a specific RNA-binding protein, both of which are known to regulate TNF-alpha gene expression at a posttranscriptional level.
Figures





Similar articles
-
Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element.Mol Cell Biol. 2006 Mar;26(6):2399-407. doi: 10.1128/MCB.26.6.2399-2407.2006. Mol Cell Biol. 2006. PMID: 16508014 Free PMC article.
-
Tristetraprolin regulates TNF TNF-alpha mRNA stability via a proteasome dependent mechanism involving the combined action of the ERK and p38 pathways.Mol Immunol. 2008 Jan;45(1):13-24. doi: 10.1016/j.molimm.2007.05.017. Epub 2007 Jul 2. Mol Immunol. 2008. PMID: 17606294
-
Decreased sensitivity of tristetraprolin-deficient cells to p38 inhibitors suggests the involvement of tristetraprolin in the p38 signaling pathway.J Biol Chem. 2001 Nov 9;276(45):42580-7. doi: 10.1074/jbc.M104953200. Epub 2001 Sep 6. J Biol Chem. 2001. PMID: 11546803 Free PMC article.
-
MAPKAP kinases MK2 and MK3 in inflammation: complex regulation of TNF biosynthesis via expression and phosphorylation of tristetraprolin.Biochem Pharmacol. 2010 Dec 15;80(12):1915-20. doi: 10.1016/j.bcp.2010.06.021. Epub 2010 Jun 23. Biochem Pharmacol. 2010. PMID: 20599781 Review.
-
AU-rich element-mediated translational control: complexity and multiple activities of trans-activating factors.Biochem Soc Trans. 2002 Nov;30(Pt 6):952-8. doi: 10.1042/bst0300952. Biochem Soc Trans. 2002. PMID: 12440953 Review.
Cited by
-
Tristetraprolin Overexpression in Non-hematopoietic Cells Protects Against Acute Lung Injury in Mice.Front Immunol. 2020 Sep 2;11:2164. doi: 10.3389/fimmu.2020.02164. eCollection 2020. Front Immunol. 2020. PMID: 32983182 Free PMC article.
-
Phosphorylation of the RNA-binding protein Dazl by MAPKAP kinase 2 regulates spermatogenesis.Mol Biol Cell. 2016 Aug 1;27(15):2341-50. doi: 10.1091/mbc.E15-11-0773. Epub 2016 Jun 8. Mol Biol Cell. 2016. PMID: 27280388 Free PMC article.
-
Apigenin prevents UVB-induced cyclooxygenase 2 expression: coupled mRNA stabilization and translational inhibition.Mol Cell Biol. 2007 Jan;27(1):283-96. doi: 10.1128/MCB.01282-06. Epub 2006 Oct 30. Mol Cell Biol. 2007. PMID: 17074806 Free PMC article.
-
Identification of the anti-inflammatory protein tristetraprolin as a hyperphosphorylated protein by mass spectrometry and site-directed mutagenesis.Biochem J. 2006 Feb 15;394(Pt 1):285-97. doi: 10.1042/BJ20051316. Biochem J. 2006. PMID: 16262601 Free PMC article.
-
Regulation of CCR4-NOT complex deadenylase activity and cellular responses by MK2-dependent phosphorylation of CNOT2.RNA Biol. 2022;19(1):234-246. doi: 10.1080/15476286.2021.2021676. Epub 2021 Dec 31. RNA Biol. 2022. PMID: 35129087 Free PMC article.
References
-
- Brook M, Sully G, Clark A R, Saklatvala J. Regulation of tumour necrosis factor alpha mRNA stability by the mitogen-activated protein kinase p38 signalling cascade. FEBS Lett. 2000;483:57–61. - PubMed
-
- Buzby J S, Brewer G, Nugent D J. Developmental regulation of RNA transcript destabilization by A+U-rich elements is AUF1-dependent. J Biol Chem. 1999;274:33973–33978. - PubMed
-
- Carballo E, Gilkeson G S, Blackshear P J. Bone marrow transplantation reproduces the tristetraprolin-deficiency syndrome in recombination activating gene-2 (−/−) mice. Evidence that monocyte/macrophage progenitors may be responsible for TNFα overproduction. J Clin Investig. 1997;100:986–995. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
