Intrachromatid excision of telomeric DNA as a mechanism for telomere size control in Saccharomyces cerevisiae
- PMID: 11533244
- PMCID: PMC99802
- DOI: 10.1128/MCB.21.19.6559-6573.2001
Intrachromatid excision of telomeric DNA as a mechanism for telomere size control in Saccharomyces cerevisiae
Abstract
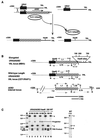
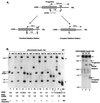
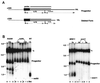
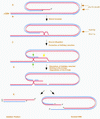
We have previously identified a process in the yeast Saccharomyces cerevisiae that results in the contraction of elongated telomeres to wild-type length within a few generations. We have termed this process telomeric rapid deletion (TRD). In this study, we use a combination of physical and genetic assays to investigate the mechanism of TRD. First, to distinguish among several recombinational and nucleolytic pathways, we developed a novel physical assay in which HaeIII restriction sites are positioned within the telomeric tract. Specific telomeres were subsequently tested for HaeIII site movement between telomeres and for HaeIII site retention during TRD. Second, genetic analyses have demonstrated that mutations in RAD50 and MRE11 inhibit TRD. TRD, however, is independent of the Rap1p C-terminal domain, a central regulator of telomere size control. Our results provide evidence that TRD is an intrachromatid deletion process in which sequences near the extreme terminus invade end-distal sequences and excise the intervening sequences. We propose that the Mre11p-Rad50p-Xrs2p complex prepares the invading telomeric overhang for strand invasion, possibly through end processing or through alterations in chromatin structure.
Figures

References
-
- Bianchi A, de Lange T. Ku binds telomeric DNA in vitro. J Biol Chem. 1999;274:21223–21227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
