Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo
- PMID: 11533707
- PMCID: PMC2818999
- DOI: 10.1038/nm0901-1035
Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo
Abstract
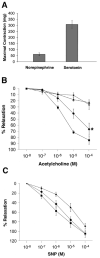
Arterial conduits are increasingly preferred for surgical bypass because of inherent functional properties conferred by arterial endothelial cells, especially nitric oxide production in response to physiologic stimuli. Here we tested whether endothelial progenitor cells (EPCs) can replace arterial endothelial cells and promote patency in tissue-engineered small-diameter blood vessels (4 mm). We isolated EPCs from peripheral blood of sheep, expanded them ex vivo and then seeded them on decellularized porcine iliac vessels. EPC-seeded grafts remained patent for 130 days as a carotid interposition graft in sheep, whereas non-seeded grafts occluded within 15 days. The EPC-explanted grafts exhibited contractile activity and nitric-oxide-mediated vascular relaxation that were similar to native carotid arteries. These results indicate that EPCs can function similarly to arterial endothelial cells and thereby confer longer vascular-graft survival. Due to their unique properties, EPCs might have other general applications for tissue-engineered structures and in treating vascular diseases.
Figures


Comment in
-
Nimble progenitors rescue vascular grafts.Nat Med. 2001 Sep;7(9):996-7. doi: 10.1038/nm0901-996b. Nat Med. 2001. PMID: 11533698 No abstract available.
References
-
- Heart and Stroke Facts: Statistical Supplement. American Heart Association; 2000. http://www.americanheart.org/statistics.
-
- Tu JV, et al. Use of cardiac procedures and outcomes in elderly patients with myocardial infarction in the United States and Cannada. N Engl J Med. 1997;336:1500. - PubMed
-
- Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;213:397. - PubMed
-
- L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47. - PubMed
-
- Niklason LE, et al. Functional arteries grown in vitro. Science. 1999;284:489. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

