Kettin, a major source of myofibrillar stiffness in Drosophila indirect flight muscle
- PMID: 11535621
- PMCID: PMC2196178
- DOI: 10.1083/jcb.200104016
Kettin, a major source of myofibrillar stiffness in Drosophila indirect flight muscle
Abstract
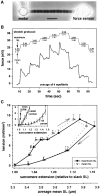
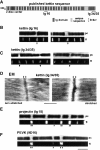
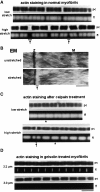
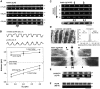
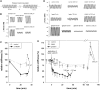
Kettin is a high molecular mass protein of insect muscle that in the sarcomeres binds to actin and alpha-actinin. To investigate kettin's functional role, we combined immunolabeling experiments with mechanical and biochemical studies on indirect flight muscle (IFM) myofibrils of Drosophila melanogaster. Micrographs of stretched IFM sarcomeres labeled with kettin antibodies revealed staining of the Z-disc periphery. After extraction of the kettin-associated actin, the A-band edges were also stained. In contrast, the staining pattern of projectin, another IFM-I-band protein, was not altered by actin removal. Force measurements were performed on single IFM myofibrils to establish the passive length-tension relationship and record passive stiffness. Stiffness decreased within seconds during gelsolin incubation and to a similar degree upon kettin digestion with mu-calpain. Immunoblotting demonstrated the presence of kettin isoforms in normal Drosophila IFM myofibrils and in myofibrils from an actin-null mutant. Dotblot analysis revealed binding of COOH-terminal kettin domains to myosin. We conclude that kettin is attached not only to actin but also to the end of the thick filament. Kettin along with projectin may constitute the elastic filament system of insect IFM and determine the muscle's high stiffness necessary for stretch activation. Possibly, the two proteins modulate myofibrillar stiffness by expressing different size isoforms.
Figures



References
-
- Benian, G.M., A. Ayme-Southgate, and T.L. Tinley. 1999. The genetics and molecular biology of the titin/connectin-like proteins of invertebrates. Rev. Physiol. Biochem. Pharmacol. 138:235–268. - PubMed
-
- Bullard, B., K.S. Hammond, and B.M. Luke. 1977. The site of paramyosin in insect flight muscle and the presence of an unidentified protein between myosin filaments and Z-line. J. Mol. Biol. 115:417–440. - PubMed
-
- Bullard, B., K. Leonard, A. Larkins, G. Butcher, C. Karlik, and E. Fyrberg. 1988. Troponin of asynchronous flight muscle. J. Mol. Biol. 294:621–637. - PubMed
-
- Bullard, B., D. Goulding, C. Ferguson, and K. Leonard. 2000. Links in the chain: the contribution of kettin to the elasticity of insect muscles. Adv. Exp. Med. Biol. 481:207–218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

