Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo
- PMID: 11535623
- PMCID: PMC2196184
- DOI: 10.1083/jcb.200103111
Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo
Erratum in
- J Cell Biol 2001 Nov 26;155(5):859. Yuen SM [corrected to Moon YS]
Abstract
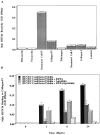
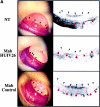
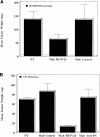
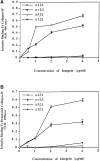
Evidence is provided that proteolytic cleavage of collagen type IV results in the exposure of a functionally important cryptic site hidden within its triple helical structure. Exposure of this cryptic site was associated with angiogenic, but not quiescent, blood vessels and was required for angiogenesis in vivo. Exposure of the HUIV26 epitope was associated with a loss of alpha1beta1 integrin binding and the gain of alphavbeta3 binding. A monoclonal antibody (HUIV26) directed to this site disrupts integrin-dependent endothelial cell interactions and potently inhibits angiogenesis and tumor growth. Together, these studies suggest a novel mechanism by which proteolysis contributes to angiogenesis by exposing hidden regulatory elements within matrix-immobilized collagen type IV.
Figures











References
-
- Blood, C.H., and B.R. Zetter. 1990. Functional interactions with the vasculature: angiogenesis and tumor metastasis. Biochim. Biophys. Acta. 1032:89–118. - PubMed
-
- Bowersox, J.C., and N. Sorgenete. 1982. Chemotaxis of aortic endothelial cells in response to fibronectin. Cancer Res. 42:2547–2551. - PubMed
-
- Brooks, P.C., A.M.P. Montgomery, M. Rosenfeld, R.A. Reisfeld, T. Hu, G. Klier, and D.A. Cheresh. 1994. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 79:1157–1164. - PubMed
-
- Brooks, P.C., S. Stromblad, L.C. Sanders, T.L. von Schalscha, R.T. Aimes, W.G. Stetler-Stevenson, J.P. Quigley, and D.A. Cheresh. 1996. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin. Cell. 85:683–693. - PubMed
-
- Brooks, P.C., A.M.P. Montgomery, and D.A. Cheresh. 1998. Use of the 10 day old chick embryo model for studying angiogenesis. A.R. Howlett, editor. Humana Press Inc., Totowa, NJ. 257–269. - PubMed

