T cell homeostasis: thymus regeneration and peripheral T cell restoration in mice with a reduced fraction of competent precursors
- PMID: 11535628
- PMCID: PMC2195939
- DOI: 10.1084/jem.194.5.591
T cell homeostasis: thymus regeneration and peripheral T cell restoration in mice with a reduced fraction of competent precursors
Abstract
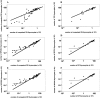
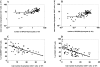
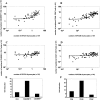
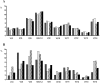
We developed a novel experimental strategy to study T cell regeneration after bone marrow transplantation. We assessed the fraction of competent precursors required to repopulate the thymus and quantified the relationship between the size of the different T cell compartments during T cell maturation in the thymus. The contribution of the thymus to the establishment and maintenance of the peripheral T cell pools was also quantified. We found that the degree of thymus restoration is determined by the availability of competent precursors and that the number of double-positive thymus cells is not under homeostatic control. In contrast, the sizes of the peripheral CD4 and CD8 T cell pools are largely independent of the number of precursors and of the number of thymus cells. Peripheral "homeostatic" proliferation and increased export and/or survival of recent thymus emigrants compensate for reduced T cell production in the thymus. In spite of these reparatory processes, mice with a reduced number of mature T cells in the thymus have an increased probability of peripheral T cell deficiency, mainly in the naive compartment.
Figures
References
-
- Mackall C.L., Hakim F.T., Gress R.E. Restoration of T-cell homeostasis after T-cell depletion. Semin. Immunol. 1997;9:339–346. - PubMed
-
- Douek D.C., Vescio R.A., Betts M.R., Brenchley J.M., Hill B.J., Zhang L., Berenson J.R., Collins R.H., Koup R.A. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355:1875–1881. - PubMed
-
- Roux E., Dumont-Girard F., Starobinski M., Siegrist C.A., Helg C., Chapuis B., Roosnek E. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood. 2000;96:2299–2303. - PubMed
-
- Cavazzana-Calvo M., Hacein-Bey S., de Saint Basile G., Gross F., Yvon E., Nusbaum P., Selz F., Hue C., Certain S., Casanova J.L. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. - PubMed
-
- Mackall C.L., Gress R.E. Thymic aging and T-cell regeneration. Immunol. Rev. 1997;160:91–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

