Suppression of Ras-mediated tumorigenicity and metastasis through inhibition of the Met receptor tyrosine kinase
- PMID: 11535809
- PMCID: PMC58533
- DOI: 10.1073/pnas.191067898
Suppression of Ras-mediated tumorigenicity and metastasis through inhibition of the Met receptor tyrosine kinase
Abstract
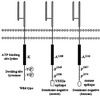
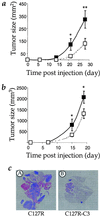
Mutations in the Ras family of GTP binding proteins represent one of the most frequently observed genetic alterations in human cancers. We and others have recently demonstrated that expression of Met, the tyrosine kinase receptor for hepatocyte growth factor/scatter factor (HGF/SF), is significantly up-regulated in Ras-transformed cells. Because HGF/SF-Met signaling is proposed to play a prominent role in tumor development and progression, we assessed the possible requirement for Met during Ras-mediated tumor growth and metastasis. To disrupt endogenous Met signaling, we constructed dominant-negative mutants of both human and murine Met and showed that these can inhibit HGF/SF-mediated Met signaling and cell invasion of ras-transformed cells in vitro. Moreover, ectopic expression of dominant-negative Met mutants reduced the s.c. tumor growth of ras-transformed cells and dramatically suppressed their ability to form lung metastases in vivo. Our data demonstrate that Met plays a prominent role during Ras-mediated tumor growth and metastasis, and further suggest that agents that inhibit HGF/SF-Met signaling may represent an important therapeutic avenue for the treatment of a variety of malignant tumors.
Figures


References
-
- Hanahan D, Weinberg R A. Cell. 2000;100:57–70. - PubMed
-
- Greenlee R, Murray T, Bolden S, Wingo P. CA Cancer J Clin. 2000;50:7–33. - PubMed
-
- Collard J G, Roos E, La, R. e G, Habets G G. Cancer Surv. 1988;7:691–710. - PubMed
-
- Bos J L. Cancer Res. 1989;49:4682–4689. - PubMed
-
- Chambers A F, Tuck A B. Crit Rev Oncog. 1993;4:95–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

