Crystal structure of the human CD4 N-terminal two-domain fragment complexed to a class II MHC molecule
- PMID: 11535811
- PMCID: PMC59561
- DOI: 10.1073/pnas.191124098
Crystal structure of the human CD4 N-terminal two-domain fragment complexed to a class II MHC molecule
Abstract
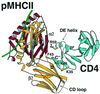
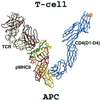
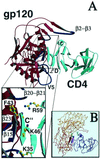
The structural basis of the interaction between the CD4 coreceptor and a class II major histocompatibility complex (MHC) is described. The crystal structure of a complex containing the human CD4 N-terminal two-domain fragment and the murine I-A(k) class II MHC molecule with associated peptide (pMHCII) shows that only the "top corner" of the CD4 molecule directly contacts pMHCII. The CD4 Phe-43 side chain extends into a hydrophobic concavity formed by MHC residues from both alpha 2 and beta 2 domains. A ternary model of the CD4-pMHCII-T-cell receptor (TCR) reveals that the complex appears V-shaped with the membrane-proximal pMHCII at the apex. This configuration excludes a direct TCR-CD4 interaction and suggests how TCR and CD4 signaling is coordinated around the antigenic pMHCII complex. Human CD4 binds to HIV gp120 in a manner strikingly similar to the way in which CD4 interacts with pMHCII. Additional contacts between gp120 and CD4 give the CD4-gp120 complex a greater affinity. Thus, ligation of the viral envelope glycoprotein to CD4 occludes the pMHCII-binding site on CD4, contributing to immunodeficiency.
Figures

References
-
- Wang J-H, Reinherz E L. Curr Opin Struct Biol. 2000;10:656–661. - PubMed
-
- Janeway C A., Jr Annu Rev Immunol. 1992;10:645–674. - PubMed
-
- Marrack P, Kappler J. Adv Immunol. 1986;38:1–30. - PubMed
-
- Gao G F, Tormo J, Gerth U C, Wyer J R, McMichael A J, Stuart D I, Bell J I, Jones E Y, Jakobsen B K. Nature (London) 1997;387:630–634. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

