Cardiomyocytes induce endothelial cells to trans-differentiate into cardiac muscle: implications for myocardium regeneration
- PMID: 11535818
- PMCID: PMC58544
- DOI: 10.1073/pnas.191217898
Cardiomyocytes induce endothelial cells to trans-differentiate into cardiac muscle: implications for myocardium regeneration
Abstract
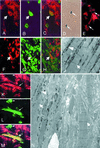
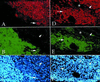
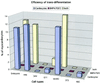
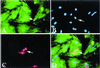
The concept of tissue-restricted differentiation of postnatal stem cells has been challenged by recent evidence showing pluripotency for hematopoietic, mesenchymal, and neural stem cells. Furthermore, rare but well documented examples exist of already differentiated cells in developing mammals that change fate and trans-differentiate into another cell type. Here, we report that endothelial cells, either freshly isolated from embryonic vessels or established as homogeneous cells in culture, differentiate into beating cardiomyocytes and express cardiac markers when cocultured with neonatal rat cardiomyocytes or when injected into postischemic adult mouse heart. Human umbilical vein endothelial cells also differentiate into cardiomyocytes under similar experimental conditions and transiently coexpress von Willebrand factor and sarcomeric myosin. In contrast, neural stem cells, which efficiently differentiate into skeletal muscle, differentiate into cardiomyocytes at a low rate. Fibroblast growth factor 2 and bone morphogenetic protein 4, which activate cardiac differentiation in embryonic cells, do not activate cardiogenesis in endothelial cells or stimulate trans-differentiation in coculture, suggesting that different signaling molecules are responsible for cardiac induction during embryogenesis and in successive periods of development. The fact that endothelial cells can generate cardiomyocytes sheds additional light on the plasticity of endothelial cells during development and opens perspectives for cell autologous replacement therapies.
Figures
Similar articles
-
Blood-borne stem cells differentiate into vascular and cardiac lineages during normal development.Stem Cells Dev. 2006 Feb;15(1):17-28. doi: 10.1089/scd.2006.15.17. Stem Cells Dev. 2006. PMID: 16522159
-
Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells.J Clin Invest. 2001 Jun;107(11):1395-402. doi: 10.1172/JCI12150. J Clin Invest. 2001. PMID: 11390421 Free PMC article.
-
Role of hepatocyte-like cells in the differentiation of cardiomyocytes from mouse embryonic stem cells.Stem Cells Dev. 2005 Apr;14(2):153-61. doi: 10.1089/scd.2005.14.153. Stem Cells Dev. 2005. PMID: 15910241
-
Non-coding RNAs in Cardiac Regeneration.Adv Exp Med Biol. 2020;1229:163-180. doi: 10.1007/978-981-15-1671-9_9. Adv Exp Med Biol. 2020. PMID: 32285411 Review.
-
Induced pluripotent stem cells as a new strategy for cardiac regeneration and disease modeling.J Mol Cell Cardiol. 2013 Sep;62:43-50. doi: 10.1016/j.yjmcc.2013.04.022. Epub 2013 Apr 30. J Mol Cell Cardiol. 2013. PMID: 23643470 Review.
Cited by
-
Targets and delivery methods for therapeutic angiogenesis in peripheral artery disease.Vasc Med. 2012 Jun;17(3):174-92. doi: 10.1177/1358863X12438270. Epub 2012 Apr 11. Vasc Med. 2012. PMID: 22496126 Free PMC article. Review.
-
Oxytocin induces differentiation of P19 embryonic stem cells to cardiomyocytes.Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9550-5. doi: 10.1073/pnas.152302499. Epub 2002 Jul 1. Proc Natl Acad Sci U S A. 2002. PMID: 12093924 Free PMC article.
-
The epigenetic reprogramming of poorly aggressive melanoma cells by a metastatic microenvironment.J Cell Mol Med. 2006 Jan-Mar;10(1):174-96. doi: 10.1111/j.1582-4934.2006.tb00299.x. J Cell Mol Med. 2006. PMID: 16563230 Free PMC article.
-
Cardiogenesis from human embryonic stem cells.Circ J. 2010 Nov;74(12):2517-26. doi: 10.1253/circj.cj-10-0958. Epub 2010 Nov 12. Circ J. 2010. PMID: 21084757 Free PMC article.
-
Hearty slices to plan for future health.Cardiovasc Res. 2009 Feb 1;81(2):235-6. doi: 10.1093/cvr/cvn347. Epub 2008 Dec 15. Cardiovasc Res. 2009. PMID: 19074822 Free PMC article. No abstract available.
References
-
- Temple S, Alavarez-Buylla A. Curr Opin Neurobiol. 1999;9:135–141. - PubMed
-
- Gage F H. Science. 2000;287:1433–1438. - PubMed
-
- Ferrari G, Cusella De Angelis M G, Coletta M, Stornaioulo A, Paolucci E, Cossu G, Mavilio F. Science. 1998;279:1528–1530. - PubMed
-
- Bjorson C R, Rietze R L, Reynolds B A, Magli M C, Vescovi A L. Science. 1999;283:534–537. - PubMed
-
- Pittenger M F, MacKay A M, Beck S C, Jaiswal R K, Douglas R, Mosca J D, Moorman M A, Simonetti D W, Craig S, Marshak D R. Science. 1999;284:143–147. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

