Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform
- PMID: 11535825
- PMCID: PMC58528
- DOI: 10.1073/pnas.191360198
Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform
Abstract
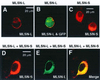
The TRP (transient receptor potential) superfamily includes a group of subfamilies of channel-like proteins mediating a multitude of physiological signaling processes. The TRP-melastatin (TRPM) subfamily includes the putative tumor suppressor melastatin (MLSN) and is a poorly characterized group of TRP-related proteins. Here, we describe the identification and characterization of an additional TRPM protein TRPM4. We reveal that TRPM4 and MLSN each mediate Ca(2+) entry when expressed in HEK293 cells. Furthermore, we demonstrate that a short form of MLSN (MLSN-S) interacts directly with and suppresses the activity of full-length MLSN (MLSN-L). This suppression seems to result from the inhibition of translocation of MLSN-L to the plasma membrane. We propose that control of translocation through interaction between MLSN-S and MLSN-L represents a mode for regulating ion channel activity.
Figures

References
-
- Harteneck C, Plant T D, Schültz G. Trends Neurosci. 2000;23:159–166. - PubMed
-
- Montell C, Rubin G M. Neuron. 1989;2:1313–1323. - PubMed
-
- Montell C. Science's STKE. 2001. http://stke.sciencemag.org/cgi/content/full/OC_sigtrans , http://stke.sciencemag.org/cgi/content/full/OC_sigtrans;2001/90/re1. ;2001/90/re1. - PubMed
-
- Hardie R C, Minke B. Neuron. 1992;8:643–651. - PubMed
-
- Freichel M, Suh S H, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, et al. Nat Cell Biol. 2001;3:121–127. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

