The yeast Xrs2 complex functions in S phase checkpoint regulation
- PMID: 11544181
- PMCID: PMC312768
- DOI: 10.1101/gad.208701
The yeast Xrs2 complex functions in S phase checkpoint regulation
Abstract
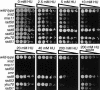
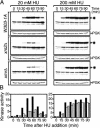
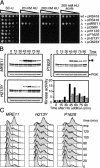
The Nbs1 complex is an evolutionarily conserved multisubunit nuclease composed of the Mre11, Rad50, and Nbs1 proteins. Hypomorphic mutations in the NBS1 or MRE11 genes in humans result in conditions characterized by DNA damage sensitivity, cell cycle checkpoint deficiency, and high cancer incidence. The equivalent complex in the yeast Saccharomyces cerevisiae (Xrs2p complex) has been implicated in DNA double-strand break repair and in telomere length regulation. Here, we find that xrs2Delta, mre11Delta, and rad50Delta mutants are markedly defective in the initiation of the intra-S phase checkpoint in response to DNA damage. Furthermore, the absence of a functional Xrs2p complex leads to sensitivity to deoxynucleotide depletion and to an inability to efficiently slow down cell cycle progression in response to hydroxyurea. The checkpoint appears to require the nuclease activity of Mre11p and its defect is associated with the abrogation of the Tel1p/Mec1p signaling pathway. Notably, DNA damage induces phosphorylation of both Xrs2p and Mre11p in a Tel1p-dependent manner. These results indicate that the Tel1p/ATM signaling pathway is conserved from yeast to humans and suggest that the Xrs2p/Nbs1 complexes act as signal modifiers.
Figures




References
-
- Allen JB, Zhou Z, Siede W, Friedberg EC, Elledge SJ. The Sad1/Rad53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes & Dev. 1994;8:2401–2415. - PubMed
-
- Carney JP, Maser RS, Olivares H, Davis EM, LeBeau M, Yates JR, Hays L, Morgan WF, Petrini JHJ. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: Linkage of double strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
