Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen
- PMID: 11544272
- PMCID: PMC209378
- DOI: 10.1172/JCI12346
Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen
Abstract
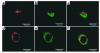
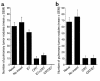
Antigen-specific cancer immunotherapy and antiangiogenesis have emerged as two attractive strategies for cancer treatment. An innovative approach that combines both mechanisms will likely generate the most potent antitumor effect. We tested this approach using calreticulin (CRT), which has demonstrated the ability to enhance MHC class I presentation and exhibit an antiangiogenic effect. We explored the linkage of CRT to a model tumor antigen, human papilloma virus type-16 (HPV-16) E7, for the development of a DNA vaccine. We found that C57BL/6 mice vaccinated intradermally with CRT/E7 DNA exhibited a dramatic increase in E7-specific CD8(+) T cell precursors and an impressive antitumor effect against E7-expressing tumors compared with mice vaccinated with wild-type E7 DNA or CRT DNA. Vaccination of CD4/CD8 double-depleted C57BL/6 mice and immunocompromised (BALB/c nu/nu) mice with CRT/E7 DNA or CRT DNA generated significant reduction of lung tumor nodules compared with wild-type E7 DNA, suggesting that antiangiogenesis may have contributed to the antitumor effect. Examination of microvessel density in lung tumor nodules and an in vivo angiogenesis assay further confirmed the antiangiogenic effect generated by CRT/E7 and CRT. Thus, cancer therapy using CRT linked to a tumor antigen holds promise for treating tumors by combining antigen-specific immunotherapy and antiangiogenesis.
Figures






References
-
- Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. - PubMed
-
- Chen CH, Wu TC. Experimental vaccine strategies for cancer immunotherapy. J Biomed Sci. 1998;5:231–252. - PubMed
-
- Folkman J. Angiogenesis: initiation and control. Ann NY Acad Sci. 1982;401:212–227. - PubMed
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. - PubMed
-
- Nash PD, Opas M, Michalak M. Calreticulin: not just another calcium-binding protein. Mol Cell Biochem. 1994;135:71–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

