Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement
- PMID: 11544274
- PMCID: PMC209379
- DOI: 10.1172/JCI12450
Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement
Abstract
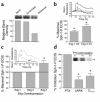
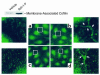
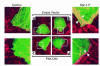
Substances released by platelets during blood clotting are essential participants in events that link hemostasis and angiogenesis and ensure adequate wound healing and tissue injury repair. We assessed the participation of sphingosine 1-phosphate (Sph-1-P), a biologically active phosphorylated lipid growth factor released from activated platelets, in the regulation of endothelial monolayer barrier integrity, which is key to both angiogenesis and vascular homeostasis. Sph-1-P produced rapid, sustained, and dose-dependent increases in transmonolayer electrical resistance (TER) across both human and bovine pulmonary artery and lung microvascular endothelial cells. This substance also reversed barrier dysfunction elicited by the edemagenic agent thrombin. Sph-1-P-mediated barrier enhancement was dependent upon G(ialpha)-receptor coupling to specific members of the endothelial differentiation gene (Edg) family of receptors (Edg-1 and Edg-3), Rho kinase and tyrosine kinase-dependent activation, and actin filament rearrangement. Sph-1-P-enhanced TER occurred in conjunction with Rac GTPase- and p21-associated kinase-dependent endothelial cortical actin assembly with recruitment of the actin filament regulatory protein, cofilin. Platelet-released Sph-1-P, linked to Rac- and Rho-dependent cytoskeletal rearrangement, may act late in angiogenesis to stabilize newly formed vessels, which often display abnormally increased vascular permeability.
Figures



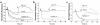




References
-
- Gimbrone MA, Jr, et al. Preservation of vascular integrity in organs perfused in vitro with a platelet-rich medium. Nature. 1969;221:33–36. - PubMed
-
- Roy AJ, Djerassi I. Effects of platelet transfusions: plug formation and maintenance of vascular integrity. Proc Soc Exp Biol Med. 1972;139:137–142. - PubMed
-
- Shepro D, Welles SL, Hechtman HB. Vasoactive agonists prevent erythrocyte extravasation in thrombocytopenic hamsters. Thromb Res. 1984;35:421–430. - PubMed
-
- Lo SK, Burhop KE, Kaplan JE, Malik AB. Role of platelets in maintenance of pulmonary vascular permeability to protein. Am J Physiol. 1988;254:H763–H771. - PubMed
-
- McDonagh PF. Platelets reduce coronary microvascular permeability to macromolecules. Am J Physiol. 1986;251:H581–H587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

