MtnK, methylthioribose kinase, is a starvation-induced protein in Bacillus subtilis
- PMID: 11545674
- PMCID: PMC55331
- DOI: 10.1186/1471-2180-1-15
MtnK, methylthioribose kinase, is a starvation-induced protein in Bacillus subtilis
Abstract
Background: Methylthioadenosine, the main by-product of spermidine synthesis, is degraded in Bacillus subtilis as adenine and methylthioribose. The latter is an excellent sulfur source and the precursor of quorum-sensing signalling molecules. Nothing was known about methylthioribose recycling in this organism.
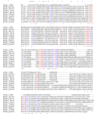
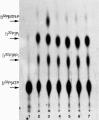
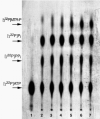
Results: Using trifluoromethylthioribose as a toxic analog to select for resistant mutants, we demonstrate that methylthioribose is first phosphorylated by MtnK, methylthioribose kinase, the product of gene mtnK (formerly ykrT), expressed as an operon with mtnS (formerly ykrS) in an abundant transcript with a S-box leader sequence. Although participating in methylthioribose recycling, the function of mtnS remained elusive. We also show that MtnK synthesis is boosted under starvation condition, in the following decreasing order: carbon-, sulfur- and nitrogen-starvation. We finally show that this enzyme is part of the family Pfam 01633 (choline kinases) which belongs to a large cluster of orthologs comprizing antibiotic aminoglycoside kinases and protein serine/threonine kinases.
Conclusions: The first step of methylthioribose recycling is phosphorylation by MTR kinase, coded by the mtnK (formerly ykrT) gene. Analysis of the neighbourhood of mtnK demonstrates that genes located in its immediate vicinity (now named mtnUVWXYZ, formerly ykrUVWXYZ) are also required for methylthioribose recycling.
Figures

Similar articles
-
Functional analysis of methylthioribose kinase genes in plants.Plant Physiol. 2004 Dec;136(4):4061-71. doi: 10.1104/pp.104.053587. Epub 2004 Nov 19. Plant Physiol. 2004. PMID: 15557090 Free PMC article.
-
Structure of Arabidopsis thaliana 5-methylthioribose kinase reveals a more occluded active site than its bacterial homolog.BMC Struct Biol. 2007 Oct 25;7:70. doi: 10.1186/1472-6807-7-70. BMC Struct Biol. 2007. PMID: 17961230 Free PMC article.
-
Selective killing of Klebsiella pneumoniae by 5-trifluoromethylthioribose. Chemotherapeutic exploitation of the enzyme 5-methylthioribose kinase.J Biol Chem. 1990 Jan 15;265(2):831-7. J Biol Chem. 1990. PMID: 2153115
-
Plausible novel ribose metabolism catalyzed by enzymes of the methionine salvage pathway in Bacillus subtilis.Biosci Biotechnol Biochem. 2013;77(5):1104-7. doi: 10.1271/bbb.120932. Epub 2013 May 7. Biosci Biotechnol Biochem. 2013. PMID: 23649237
-
Metabolic characteristics and importance of the universal methionine salvage pathway recycling methionine from 5'-methylthioadenosine.IUBMB Life. 2009 Dec;61(12):1132-42. doi: 10.1002/iub.278. IUBMB Life. 2009. PMID: 19946895 Review.
Cited by
-
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis.J Bacteriol. 2003 Apr;185(8):2418-31. doi: 10.1128/JB.185.8.2418-2431.2003. J Bacteriol. 2003. PMID: 12670965 Free PMC article.
-
Functional analysis of methylthioribose kinase genes in plants.Plant Physiol. 2004 Dec;136(4):4061-71. doi: 10.1104/pp.104.053587. Epub 2004 Nov 19. Plant Physiol. 2004. PMID: 15557090 Free PMC article.
-
A bifunctional salvage pathway for two distinct S-adenosylmethionine by-products that is widespread in bacteria, including pathogenic Escherichia coli.Mol Microbiol. 2020 May;113(5):923-937. doi: 10.1111/mmi.14459. Epub 2020 Feb 20. Mol Microbiol. 2020. PMID: 31950558 Free PMC article.
-
The methionine salvage pathway in Bacillus subtilis.BMC Microbiol. 2002 Apr 25;2:8. doi: 10.1186/1471-2180-2-8. BMC Microbiol. 2002. PMID: 12022921 Free PMC article.
-
Structure of Arabidopsis thaliana 5-methylthioribose kinase reveals a more occluded active site than its bacterial homolog.BMC Struct Biol. 2007 Oct 25;7:70. doi: 10.1186/1472-6807-7-70. BMC Struct Biol. 2007. PMID: 17961230 Free PMC article.
References
-
- Coppée JY, Auger S, Turlin E, Sekowska A, Le Caer JP, Labas V, Vagner V, Danchin A, Martin-Verstraete I. Sulfur-limitation-regulated proteins in Bacillus subtilis : a two-dimensional gel electrophoresis study. Microbiology. 2001;147:1631–1640. - PubMed
-
- Sekowska A, Coppée JY, Le Caer JP, Martin-Verstraete I, Danchin A. S-adenosylmethionine decarboxylase of Bacillus subtilis is closely related to archaebacterial counterparts. Mol Microbiol. 2000;36:1135–1147. - PubMed
-
- Sekowska A, Danchin A. Identification of yrrU as the methylthioadenosine nucleosidase gene in Bacillus subtilis. DNA Res. 1999;6:255–264. - PubMed
-
- Schroeder HR, Barnes CJ, Bohinski RC, Mallette MF. Biological production of 5-methylthioribose. Can J Microbiol. 1973;19:1347–1354. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

