Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers
- PMID: 11549579
- PMCID: PMC1850445
- DOI: 10.1016/S0002-9440(10)61762-2
Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers
Abstract
Autoimmune thyroid disease--Hashimoto thyroiditis and Graves' disease--patients produce high levels of thyroid autoantibodies and contain lymphoid tissue that resembles secondary lymphoid follicles (LFs). We compared the specificity, structure, and function of tonsil and lymph node LFs with those of the intrathyroidal LFs to assess the latter's capability to contribute to autoimmune response. Thyroglobulin and thyroperoxidase binding to LFs indicated that most intrathyroidal LFs were committed to response to thyroid self-antigens and were associated to higher levels of antibodies to thyroglobulin, thyroperoxidase, and thyroid-stimulating hormone receptor. Intrathyroidal LFs were microanatomically very similar to canonical LFs, ie, they had well-developed germinal centers with mantle, light, and dark zones and each of these zones contained B and T lymphocytes, follicular dendritic and interdigitating dendritic cells with typical phenotypes. Careful assessment of proliferation (Ki67) and apoptosis (terminal dUTP nick-end labeling) indicators and of the occurrence of secondary immunoglobulin gene rearrangements (RAG1 and RAG2) confirmed the parallelism. Unexpected high levels of RAG expression suggested that receptor revision occurs in intrathyroidal LFs and may contribute to generate high-affinity thyroid autoantibodies. Well-formed high endothelial venules and a congruent pattern of adhesion molecules and chemokine expression in intrathyroidal LFs were also detected. These data suggest that ectopic intrathyroidal LFs contain all of the elements needed to drive the autoimmune response and also that their microenvironment may favor the expansion and perpetuation of autoimmune response.
Figures


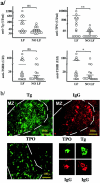


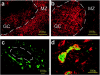
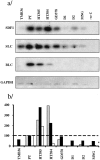
Comment in
-
Ectopic lymphoid organogenesis: a fast track for autoimmunity.Am J Pathol. 2001 Sep;159(3):787-93. doi: 10.1016/S0002-9440(10)61751-8. Am J Pathol. 2001. PMID: 11549568 Free PMC article. Review. No abstract available.
References
-
- Weetman AP, McGregor AM: Autoimmune thyroid disease: further developments in our understanding. Endocr Rev 1994, 15:788-830 - PubMed
-
- Ajjan RA, Kemp EH, Waterman EA, Watson PF, Endo T, Onaya T, Weetman AP: Detection of binding and blocking autoantibodies to the human sodium-iodide symporter in patients with autoimmune thyroid disease. J Clin Endocrinol Metab 2000, 85:2020-2027 - PubMed
-
- Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM: The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies Endocr Rev 1998, 19:673-716 - PubMed
-
- Fukuma N, Petersen VB, McLachlan SM, Pegg CA, Rees Smith B: Human monoclonal thyroglobulin autoantibodies of high affinity. I. Production, characterisation and interaction with murine monoclonal thyroglobulin antibodies. Autoimmunity 1991, 10:291-295 - PubMed
-
- Prentice L, Kiso Y, Fukuma N, Horimoto M, Petersen V, Grennan F, Pegg C, Furmaniak J, Rees Smith B: Monoclonal thyroglobulin autoantibodies: variable region analysis and epitope recognition. J Clin Endocrinol Metab 1995, 80:977-986 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

