Interleukin-1beta promotes repair of the CNS
- PMID: 11549714
- PMCID: PMC6762979
- DOI: 10.1523/JNEUROSCI.21-18-07046.2001
Interleukin-1beta promotes repair of the CNS
Abstract
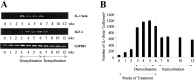
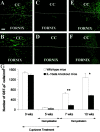
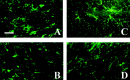
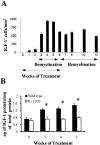
Interleukin-1beta (IL-1beta) is a proinflammatory cytokine associated with the pathophysiology of demyelinating disorders such as multiple sclerosis and viral infections of the CNS. However, we demonstrate here that IL-1beta appears to promote remyelination in the adult CNS. In IL-1beta(-/-) mice, acute demyelination progressed similarly to wild-type mice and showed parallel mature oligodendrocyte depletion, microglia-macrophage accumulation, and the appearance of oligodendrocyte precursors. In contrast, IL-1beta(-/-) mice failed to remyelinate properly, and this appeared to correlate with a lack of insulin-like growth factor-1 (IGF-1) production by microglia-macrophages and astrocytes and to a profound delay of precursors to differentiate into mature oligodendrocytes. Thus, IL-1beta may be crucial to the repair of the CNS, presumably through the induction of astrocyte and microglia-macrophage-derived IGF-1.
Figures


References
-
- Arkins S, Rebeiz N, Biragyn A, Reese DL, Kelley KW. Murine macrophages express abundant insulin-like growth factor-1 class I Ea and Eb transcripts. Endocrinology. 1993;133:2334–2343. - PubMed
-
- Bauer J, Berkenbosch F, Van Dam A-M, Dijkstra CD. Demonstration of interleukin-1β in Lewis rat brain during experimental allergic encephalomyelitis by immunohistochemistry at the light and ultrastructural level. J Neuroimmunol. 1993;48:13–22. - PubMed
-
- Bencsik A, Malcus C, Akaoka H, Giraudon P, Belin M-F, Bernard A. Selective induction of cytokines in mouse brain infected with canine distemper virus: structural, cellular and temporal expression. J Neuroimmunol. 1996;65:1–9. - PubMed
-
- Blakemore WF. Remyelination of the superior cerebellar peduncle in the mouse following demyelination induced by feeding cuprizone. J Neurol Sci. 1973;20:73–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
