Caspase 3 deficiency rescues peripheral nervous system defect in retinoblastoma nullizygous mice
- PMID: 11549719
- PMCID: PMC6762980
- DOI: 10.1523/JNEUROSCI.21-18-07089.2001
Caspase 3 deficiency rescues peripheral nervous system defect in retinoblastoma nullizygous mice
Abstract
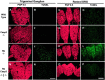
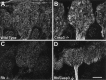
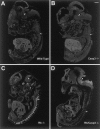
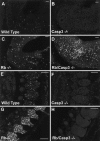
The retinoblastoma tumor suppressor protein, pRb, is a key regulator of cell cycle and has been implicated in the terminal differentiation of neuronal cells. Mice nullizygous for pRb die by embryonic day 14.5 from hematopoietic and neurological defects attributed to failed differentiation (Clarke et al., 1992; Jacks et al., 1992; Lee et al., 1992). Previous studies by MacLeod et al. (1996) have demonstrated that the loss of p53 protects Rb-deficient CNS neurons but not peripheral nervous system (PNS) neurons from cell death. Thus, the mechanisms by which PNS neurons undergo apoptosis in response to Rb deficiency remain unknown. In view of the pivotal role of caspase 3 in the regulation of neuronal apoptosis during development, we examined its function in the execution of the wide-spread neuronal cell death induced by Rb deficiency. Our results support a number of conclusions. First, we show that caspase 3 becomes activated in all neuronal populations undergoing apoptosis. Second, caspase 3 deficiency does not extend the life span of Rb null embryos, because double null mutants exhibit high rates of liver apoptosis resulting in erythropoietic failure. Third, Rb/caspase 3 double-mutant neurons of the CNS exhibit widespread apoptosis similar to that seen in Rb mutants alone; thus caspase 3 deficiency does not protect this population from apoptosis. Finally, in contrast to the CNS, neurons of the PNS including those comprising the trigeminal ganglia and the dorsal root ganglia are protected from apoptosis in Rb/caspase 3 double-mutant embryos. Examination of the mechanistic differences between these two cell types suggest that CNS neurons may invoke other caspases to facilitate apoptosis in the absence of caspase 3. These findings suggest that PNS neurons are dependent on caspase 3 for the execution of apoptosis and that caspase 3 may serve as a key therapeutic target for neuroprotection after injury of this cell type.
Figures






References
-
- Caccamo D, Katsetos CD, Herman MM, Frankfurter A, Collins VP, Rubinstein LJ. Immunohistochemistry of a spontaneous murine ovarian teratoma with neuroepithelial differentiation. Lab Invest. 1989;60:390–398. - PubMed
-
- Callaghan DA, Dong L, Callaghan SM, Hou YX, Dagnino L, Slack RS. Neural precursor cells differentiating in the absence of Rb exhibit delayed terminal mitosis and deregulated E2F1 and 3 activity. Dev Biol. 1999;207:257–270. - PubMed
-
- Clarke AR, Maandag ER, Vam Roon M, Van der Lugt NMT, Van der Valk M, Hooper MI, Berns A, Te Reile H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992;359:328–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
