The Drosophila double-timeS mutation delays the nuclear accumulation of period protein and affects the feedback regulation of period mRNA
- PMID: 11549722
- PMCID: PMC6762998
- DOI: 10.1523/JNEUROSCI.21-18-07117.2001
The Drosophila double-timeS mutation delays the nuclear accumulation of period protein and affects the feedback regulation of period mRNA
Abstract
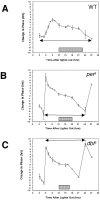
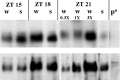
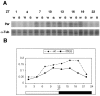
The Drosophila double-time (dbt) gene, which encodes a protein similar to vertebrate epsilon and delta isoforms of casein kinase I, is essential for circadian rhythmicity because it regulates the phosphorylation and stability of period (per) protein. Here, the circadian phenotype of a short-period dbt mutant allele (dbt(S)) was examined. The circadian period of the dbt(S) locomotor activity rhythm varied little when tested at constant temperatures ranging from 20 to 29 degrees C. However, per(L);dbt(S) flies exhibited a lack of temperature compensation like that of the long-period mutant (per(L)) flies. Light-pulse phase-response curves were obtained for wild-type, the short-period (per(S)), and dbt(S) genotypes. For the per(S) and dbt(S) genotypes, phase changes were larger than those for wild-type flies, the transition period from delays to advances was shorter, and the light-insensitive period was shorter. Immunohistochemical analysis of per protein levels demonstrated that per protein accumulates in photoreceptor nuclei later in dbt(S) than in wild-type and per(S) flies, and that it declines to lower levels in nuclei of dbt(S) flies than in nuclei of wild-type flies. Immunoblot analysis of per protein levels demonstrated that total per protein accumulation in dbt(S) heads is neither delayed nor reduced, whereas RNase protection analysis demonstrated that per mRNA accumulates later and declines sooner in dbt(S) heads than in wild-type heads. These results suggest that dbt can regulate the feedback of per protein on its mRNA by delaying the time at which it is translocated to nuclei and altering the level of nuclear PER during the declining phase of the cycle.
Figures


References
-
- Baylies MK, Bargiello TA, Jackson FR, Young MW. Changes in abundance or structure of the per gene product can alter periodicity of the Drosophila clock. Nature. 1987;326:390–392. - PubMed
-
- Cermakian N, Sassone-Corsi P. Multilevel regulation and the circadian clock. Nat Rev Mol Cell Biol. 2000;1:59–67. - PubMed
-
- Curtin KD, Huang ZJ, Rosbash M. Temporally regulated nuclear entry of the Drosophila period protein contributes to the circadian clock. Neuron. 1995;14:365–372. - PubMed
-
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
