The Meissner corpuscle revised: a multiafferented mechanoreceptor with nociceptor immunochemical properties
- PMID: 11549734
- PMCID: PMC6763005
- DOI: 10.1523/JNEUROSCI.21-18-07236.2001
The Meissner corpuscle revised: a multiafferented mechanoreceptor with nociceptor immunochemical properties
Abstract
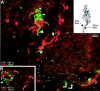
Meissner corpuscles (MCs) in the glabrous skin of monkey digits have at least three types of innervation as revealed by immunofluorescence. The previously well known Aalphabeta-fiber terminals are closely intertwined with endings from peptidergic C-fibers. These intertwined endings are segregated into zones that alternate with zones containing a third type of ending supplied by nonpeptidergic C-fibers. Although MCs are widely regarded as low-threshold mechanoreceptors, all three types of innervation express immunochemical properties associated with nociception. The peptidergic C-fiber endings have readily detectable levels of immunoreactivity (IR) for calcitonin gene-related peptide (CGRP) and substance P (SP). The Aalphabeta endings have relatively lower levels of IR for CGRP and SP as well as the SP neurokinin 1 receptor and vanilloid-like receptor 1. Both the Aalphabeta and peptidergic C-fiber endings were also labeled with antibodies for different combinations of adrenergic, opioid, and purinergic receptors. The nonpeptidergic C-fiber endings express IR for vanilloid receptor 1, which has also been implicated in nociception. Thus, MCs are multiafferented receptor organs that may have nociceptive capabilities in addition to being low-threshold mechanoreceptors.
Figures



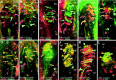
References
-
- Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee J-H, Nakano AH, Lin X, Loh HH, Law P-Y, Wessendorf MW, Elde R. The κ-opioid receptor (KOR1) is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proc Natl Acad Sci USA. 1995c;92:5062–5066. - PMC - PubMed
-
- Ballet S, Mauborgne A, Benoliel J-J, Bourgoin S, Hamon M, Cesselin F, Collin E. Polyarthritis-associated changes in the opioid control of spinal CGRP release in the rat. Brain Res. 1998;796:198–208. - PubMed
-
- Björklund H, Dalsgaard CJ, Jonsson C-E, Hermasson A. Sensory autonomic innervation of non-hairy skin and hairy human skin. Cell Tissue Res. 1986;243:51–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
