Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat
- PMID: 11549737
- PMCID: PMC6762996
- DOI: 10.1523/JNEUROSCI.21-18-07273.2001
Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat
Abstract
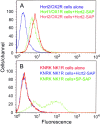
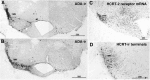
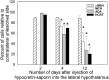
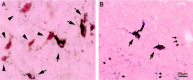
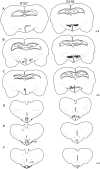
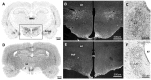
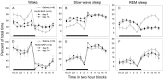
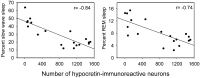
Hypocretins (Hcrts) are recently discovered peptides linked to the human sleep disorder narcolepsy. Humans with narcolepsy have decreased numbers of Hcrt neurons and Hcrt-null mice also have narcoleptic symptoms. Hcrt neurons are located only in the lateral hypothalamus (LH) but neither electrolytic nor pharmacological lesions of this or any other brain region have produced narcoleptic-like sleep, suggesting that specific neurons need to be destroyed. Hcrt neurons express the Hcrt receptor, and to facilitate lesioning these neurons, the endogenous ligand hypocretin-2/orexin B (Hcrt2) was conjugated to the ribosome-inactivating protein saporin (SAP). In vitro binding studies indicated specificity of the Hcrt2-SAP because it preferentially bound to Chinese hamster ovary cells containing the Hcrt/orexin receptor 2 (HcrtR2/OX(2)R) or the Hcrt/orexin receptor 1 (HcrtR1/OX(1)R) but not to Kirsten murine sarcoma virus transformed rat kidney epithelial (KNRK) cells stably transfected with the substance P (neurokinin-1) receptor. Administration of the toxin to the LH, in which the receptor is known to be present, eliminated some neurons (Hcrt, melanin-concentrating hormone, and adenosine deaminase-containing neurons) but not others (a-melanocyte-stimulating hormone), indicating specificity of the toxin in vivo. When the toxin was administered to the LH, rats had increased slow-wave sleep, rapid-eye movement (REM) sleep, and sleep-onset REM sleep periods. These behavioral changes were negatively correlated with the loss of Hcrt-containing neurons but not with the loss of adenosine deaminase-immunoreactive neurons. These findings indicate that damage to the LH that also causes a substantial loss of Hcrt neurons is likely to produce the multiple sleep disturbances that occur in narcolepsy.
Figures

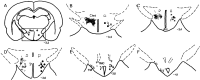
References
-
- Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889:1–22. - PubMed
-
- Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. NeuroReport. 2001;12:435–440. - PubMed
-
- Aldrich MS. The neurobiology of narcolepsy. Trends Neurosci. 1991;14:235–239. - PubMed
-
- Bergamaschi G, Perfetti V, Tonon L, Novella A, Lucotti C, Danova M, Glennie MJ, Merlini G, Cazzola M. Saporin, a ribosome-inactivating protein used to prepare immunotoxins, induces cell death via apoptosis. Br J Haematol. 1996;93:789–794. - PubMed
-
- Borbely AA. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Saunders; Toronto: 1994. pp. 309–320.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
