Viruses of lower vertebrates
- PMID: 11550762
- PMCID: PMC7159363
- DOI: 10.1046/j.1439-0450.2001.00473.x
Viruses of lower vertebrates
Abstract
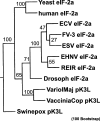
Viruses of lower vertebrates recently became a field of interest to the public due to increasing epizootics and economic losses of poikilothermic animals. These were reported worldwide from both wildlife and collections of aquatic poikilothermic animals. Several RNA and DNA viruses infecting fish, amphibians and reptiles have been studied intensively during the last 20 years. Many of these viruses induce diseases resulting in important economic losses of lower vertebrates, especially in fish aquaculture. In addition, some of the DNA viruses seem to be emerging pathogens involved in the worldwide decline in wildlife. Irido-, herpes- and polyomavirus infections may be involved in the reduction in the numbers of endangered amphibian and reptile species. In this context the knowledge of several important RNA viruses such as orthomyxo-, paramyxo-, rhabdo-, retro-, corona-, calici-, toga-, picorna-, noda-, reo- and birnaviruses, and DNA viruses such as parvo-, irido-, herpes-, adeno-, polyoma- and poxviruses, is described in this review.
Figures

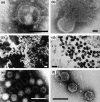


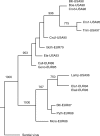
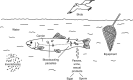
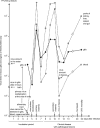


References
-
- Adkison, A. M. , Cambre M., Hedrick R. P., 1998: Identification of an iridovirus in Russian sturgeon (Acipenser guldenstadi) from northern Europe. Bull. Eur. Assoc. Fish Pathol. 18 , 29–32.
-
- Aguirre, A. A. , Spraker T. R., Chaves A., DuToit L., Eure W., Balazs G. H., 1999: Pathology of fibropapillomatosis in olive ridley turtles Lepidochelys olivacea nesting in Costa Rica. J. Aquat. Anim. Health 11 , 283–289.
-
- Ahne, W. , 1978a: Uptake and multiplication of spring viremia of carp virus in carp (Cyprinus carpio). J. Fish Dis. 1 , 265–268.
-
- Ahne, W. , 1978b: Isolation and characterization of infectious pancreatic necrosis virus from pike (Esox lucius). Arch. Virol. 58 , 65–69. - PubMed
-
- Ahne, W. , 1985a: Argulus foliaceus L., and Piscicola geometra L. as mechanical vectors of spring viraemia of carp virus (SVCV). J. Fish Dis. 8 , 241–242.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

