Release of Toll-like receptor-2-activating bacterial lipoproteins in Shigella flexneri culture supernatants
- PMID: 11553567
- PMCID: PMC98758
- DOI: 10.1128/IAI.69.10.6248-6255.2001
Release of Toll-like receptor-2-activating bacterial lipoproteins in Shigella flexneri culture supernatants
Abstract
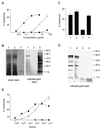
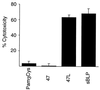
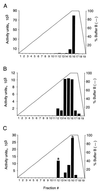
Shigella spp. cause dysentery, a severe form of bloody diarrhea. Apoptosis, or programmed cell death, is induced during Shigella infections and has been proposed to be a key event in the pathogenesis of dysentery. Here, we describe a novel cytotoxic activity in the sterile-culture supernatants of Shigella flexneri. An identical activity was identified in purified S. flexneri endotoxin, defined here as a mixture of lipopolysaccharide (LPS) and endotoxin-associated proteins (EP). Separation of endotoxin into EP and LPS revealed the activity to partition exclusively to the EP fraction. Biochemical characterization of S. flexneri EP and culture supernatants, including enzymatic deactivation, reverse-phase high-pressure liquid chromatography analysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and a Toll-like receptor-2 (TLR2) activation assay, indicates that the cytotoxic component is a mixture of bacterial lipoproteins (BLP). We show that biologically active BLP are liberated into culture supernatants of actively growing S. flexneri. In addition, our data indicate that BLP, and not LPS, are the component of endotoxin of gram-negative organisms responsible for activating TLR2. The activation of apoptosis by BLP shed from S. flexneri is discussed as a novel aspect of the interaction of bacteria with the host.
Figures
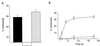
References
-
- Aliprantis A O, Yang R B, Mark M R, Suggett S, Devaux B, Radolf J D, Klimpel G R, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. - PubMed
-
- Baeuerle P, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
-
- Brightbill H D, Libraty D H, Krutzik S R, Belisle J T, Maitland M, Norgard M V, Plevy S E, Smale S T, Brennan P J, Bloom B R, Godowski P J, Modlin R L. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

