Macrophage nitric oxide synthase associates with cortical actin but is not recruited to phagosomes
- PMID: 11553583
- PMCID: PMC98774
- DOI: 10.1128/IAI.69.10.6391-6400.2001
Macrophage nitric oxide synthase associates with cortical actin but is not recruited to phagosomes
Abstract
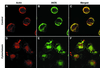
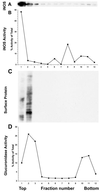
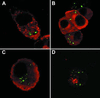
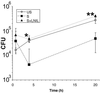
Nitric oxide (NO) produced from inducible NO synthase (iNOS) is an important component of host defense against intracellular pathogens. To understand how phagocytes deliver NO to ingested microorganisms while avoiding cytotoxicity, we set out to study the subcellular localization of iNOS within macrophages following phagocytosis. Confocal microscopy of immunostained cells showed that iNOS was located not only diffusely within cytoplasm but also in vesicles, as well as immediately adjacent to the peripheral cell membrane. This peripheral iNOS colocalized with the cortical actin cytoskeleton and was removed by the actin-depolymerizing drug cytochalasin B. Biochemical fractionation of RAW 264 macrophages showed that 32.75% (+/-5.11%; n = 3) of iNOS was present in a particulate fraction, which cosedimented with low-density cellular vesicles. Following phagocytosis of latex beads, zymosan, immunoglobulin G-coated beads, or complement-coated zymosan, submembranous cortical iNOS was not recruited to phagosomes, nor was there any relocalization of intracellular iNOS. Similarly, following phagocytosis of Salmonella enterica serovar Typhimurium there was no recruitment of iNOS to the Salmonella vacuole at any stage after internalization. NO mediated significant killing of intracellular S. enterica serovar Typhimurium in RAW macrophages treated with lipopolysaccharide and gamma interferon; this was evident 4 h after infection. Although not recruited to phagosomes, iNOS association with the submembranous cortical actin cytoskeleton is ideally suited to deliver NO to microbes in contact with the cell surface and may contribute to early killing of ingested Salmonella.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

