Cyclophosphamide decreases nitrotyrosine formation and inhibits nitric oxide production by alveolar macrophages in mycoplasmosis
- PMID: 11553584
- PMCID: PMC98775
- DOI: 10.1128/IAI.69.10.6401-6410.2001
Cyclophosphamide decreases nitrotyrosine formation and inhibits nitric oxide production by alveolar macrophages in mycoplasmosis
Abstract
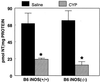
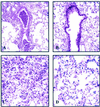
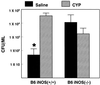
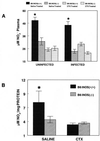
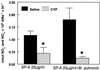
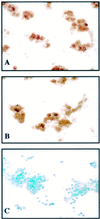
We previously reported that congenic C57BL/6 inducible nitric oxide synthase(-/-) (iNOS(-/-)) mice infected with Mycoplasma pulmonis developed higher bacterial numbers and lung lesion scores than C57BL/6 iNOS(+/+) controls but had similar lung nitrotyrosine levels. The present studies investigated the role of inflammatory cells in nitrotyrosine formation during mycoplasmal infection. iNOS(+/+) and iNOS(-/-) mice were injected with cyclophosphamide (CYP) and inoculated with 10(7) CFU of M. pulmonis. CYP pretreatment of M. pulmonis-infected iNOS(+/+) and iNOS(-/-) mice reduced polymorphonuclear cells (PMNs) within bronchoalveolar lavages (BALs) by 88 and 72%, respectively, and whole-lung myeloperoxidase levels by 80 and 78%, respectively, at 72 h postinfection but did not alter the number of alveolar macrophages (AMs) in BALs. CYP treatment also significantly decreased nitrate and nitrite (NOx) levels in BALs and plasma of infected iNOS(+/+) mice, whereas neither CYP nor mycoplasmal infection altered NOx in iNOS(-/-) mice. CYP reduced lung nitrotyrosine levels in both iNOS(+/+) and iNOS(-/-) mice to uninfected-control levels as shown by immunohistochemical staining and enzyme-linked immunosorbent assay and inhibited mycoplasmal killing by iNOS(+/+) mice in vivo. CYP inhibited the production of gamma interferon-inducible NOx by iNOS(+/+) AMs in vitro but did not alter the number of iNOS-positive AMs, as detected by immunocytochemistry. In addition, AMs from CYP-treated iNOS(+/+) mice had significantly decreased ability to kill mycoplasmas in vitro. These results demonstrate that reactive species generated by inflammatory cells as well as PMN myeloperoxidase are important contributors to nitrotyrosine formation during mycoplasmal infection and that treatment with CYP decreases NO* production by AMs and inhibits mycoplasmal killing.
Figures




Similar articles
-
Response of alveolar macrophages from inducible nitric oxide synthase knockout or wild-type mice to an in vitro lipopolysaccharide or silica exposure.J Toxicol Environ Health A. 2003 Jun 13;66(11):995-1013. doi: 10.1080/15287390306395. J Toxicol Environ Health A. 2003. PMID: 12775513
-
Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages by production of peroxynitrite.Proc Natl Acad Sci U S A. 1999 Apr 27;96(9):4953-8. doi: 10.1073/pnas.96.9.4953. Proc Natl Acad Sci U S A. 1999. PMID: 10220400 Free PMC article.
-
Murine respiratory mycoplasmosis: a model to study effects of oxidants.Res Rep Health Eff Inst. 1991 Dec;(47):1-29; discussion 31-43. Res Rep Health Eff Inst. 1991. PMID: 1838926
-
Depletion of alveolar macrophages exacerbates respiratory mycoplasmosis in mycoplasma-resistant C57BL mice but not mycoplasma-susceptible C3H mice.Infect Immun. 1997 Jun;65(6):2278-82. doi: 10.1128/iai.65.6.2278-2282.1997. Infect Immun. 1997. PMID: 9169764 Free PMC article.
-
Nitric oxide production by rat bronchoalveolar macrophages or polymorphonuclear leukocytes following intratracheal instillation of lipopolysaccharide or silica.J Biosci. 2003 Feb;28(1):29-37. doi: 10.1007/BF02970129. J Biosci. 2003. PMID: 12682422
Cited by
-
A novel IL-17-dependent mechanism of cross protection: respiratory infection with mycoplasma protects against a secondary listeria infection.Eur J Immunol. 2009 Feb;39(2):426-38. doi: 10.1002/eji.200838726. Eur J Immunol. 2009. PMID: 19180464 Free PMC article.
-
Surfactant dysfunction in SP-A-/- and iNOS-/- mice with mycoplasma infection.Am J Respir Cell Mol Biol. 2007 Jan;36(1):103-13. doi: 10.1165/rcmb.2006-0049OC. Epub 2006 Aug 17. Am J Respir Cell Mol Biol. 2007. PMID: 16917077 Free PMC article.
-
Elevated generation of reactive oxygen/nitrogen species in hantavirus cardiopulmonary syndrome.J Virol. 2002 Aug;76(16):8347-59. doi: 10.1128/jvi.76.16.8347-8359.2002. J Virol. 2002. PMID: 12134039 Free PMC article.
-
Synergistic effects of herpes oncolytic virus and cyclophosphamide for recurrent malignant glioma: a narrative review.Ann Med Surg (Lond). 2024 Jul 17;86(9):5354-5360. doi: 10.1097/MS9.0000000000002384. eCollection 2024 Sep. Ann Med Surg (Lond). 2024. PMID: 39239066 Free PMC article. Review.
-
Protective effects of Zhuyeqing liquor on the immune function of normal and immunosuppressed mice in vivo.BMC Complement Altern Med. 2013 Oct 3;13:252. doi: 10.1186/1472-6882-13-252. BMC Complement Altern Med. 2013. PMID: 24090456 Free PMC article.
References
-
- Anderson D, Bishop J B, Garner R C, Ostrosky-Wegman P, Selby P B. Cyclophosphamide: review of its mutagenicity for an assessment of potential germ cell risks. Mutat Res. 1995;330:115–181. - PubMed
-
- Beckman J S, Ye Y Z, Anderson P G, Chen J, Accavitti M A, Tarpey M M, White C R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe-Seyler. 1994;375:81–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

