Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae
- PMID: 11553605
- PMCID: PMC98796
- DOI: 10.1128/IAI.69.10.6549-6553.2001
Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae
Abstract
Vibrio cholerae hemagglutinin/protease (Hap) was induced upon nutrient limitation and strongly repressed by glucose. Hap was not produced in a mutant defective in the cyclic AMP (cAMP) receptor protein, suggesting that intracellular cAMP levels mediate Hap expression. No difference was found in Hap production between an rpoS deletion mutant and its isogenic wild-type precursor, indicating that the alternate sigma(s) factor is not essential for Hap expression. Based on these and previous results, we discuss the role of Hap in the pathogenesis of cholera.
Figures

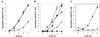

References
-
- Benitez J A, Garcia L, Silva A, Garcia H, Fando R, Cedré B, Pérez A, Campos J, Rodriguez B L, Pérez J L, Valmaseda T, Pérez O, Pérez A, Ramírez M, Ledón T, Díaz Jidy M, Lastre M, Bravo L, Sierra G. Preliminary assessment of the safety and immunogenicity of a new CTXΦ-negative hemagglutinin/protease-defective El Tor strain as a cholera vaccine candidate. Infect Immun. 1999;67:539–545. - PMC - PubMed
-
- Ferraris R P, Yasharpour S, Lloyd K C, Mirzayan R, Diamond J M. Luminal glucose concentrations in the gut under normal conditions. Am J Physiol. 1990;259:G822–G837. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

