Myosin Va bound to phagosomes binds to F-actin and delays microtubule-dependent motility
- PMID: 11553713
- PMCID: PMC59709
- DOI: 10.1091/mbc.12.9.2742
Myosin Va bound to phagosomes binds to F-actin and delays microtubule-dependent motility
Abstract
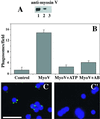
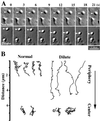
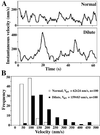
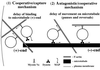
We established a light microscopy-based assay that reconstitutes the binding of phagosomes purified from mouse macrophages to preassembled F-actin in vitro. Both endogenous myosin Va from mouse macrophages and exogenous myosin Va from chicken brain stimulated the phagosome-F-actin interaction. Myosin Va association with phagosomes correlated with their ability to bind F-actin in an ATP-regulated manner and antibodies to myosin Va specifically blocked the ATP-sensitive phagosome binding to F-actin. The uptake and retrograde transport of phagosomes from the periphery to the center of cells in bone marrow macrophages was observed in both normal mice and mice homozygous for the dilute-lethal spontaneous mutation (myosin Va null). However, in dilute-lethal macrophages the accumulation of phagosomes in the perinuclear region occurred twofold faster than in normal macrophages. Motion analysis revealed saltatory phagosome movement with temporarily reversed direction in normal macrophages, whereas almost no reversals in direction were observed in dilute-lethal macrophages. These observations demonstrate that myosin Va mediates phagosome binding to F-actin, resulting in a delay in microtubule-dependent retrograde phagosome movement toward the cell center. We propose an "antagonistic/cooperative mechanism" to explain the saltatory phagosome movement toward the cell center in normal macrophages.
Figures




Similar articles
-
Myosin-Va binds to and mechanochemically couples microtubules to actin filaments.Mol Biol Cell. 2004 Jan;15(1):151-61. doi: 10.1091/mbc.e03-07-0504. Epub 2003 Oct 17. Mol Biol Cell. 2004. PMID: 14565972 Free PMC article.
-
High affinity binding of brain myosin-Va to F-actin induced by calcium in the presence of ATP.J Biol Chem. 2001 Oct 26;276(43):39812-8. doi: 10.1074/jbc.M102583200. Epub 2001 Aug 21. J Biol Chem. 2001. PMID: 11517216
-
Missense mutations in the globular tail of myosin-Va in dilute mice partially impair binding of Slac2-a/melanophilin.J Cell Sci. 2004 Feb 1;117(Pt 4):583-91. doi: 10.1242/jcs.00891. J Cell Sci. 2004. PMID: 14730011
-
Role of microtubules and myosins in Fc gamma receptor-mediated phagocytosis.Front Biosci. 2006 May 1;11:1479-90. doi: 10.2741/1897. Front Biosci. 2006. PMID: 16368530 Review.
-
The melanosome as a model to study organelle motility in mammals.Pigment Cell Res. 2004 Apr;17(2):111-8. doi: 10.1111/j.1600-0749.2004.00138.x. Pigment Cell Res. 2004. PMID: 15016299 Review.
Cited by
-
Radiosensitizing effect of epothilone B on human epithelial cancer cells.Strahlenther Onkol. 2012 Feb;188(2):177-84. doi: 10.1007/s00066-011-0029-4. Epub 2012 Jan 12. Strahlenther Onkol. 2012. PMID: 22234539
-
Spatio-temporal model of endogenous ROS and raft-dependent WNT/beta-catenin signaling driving cell fate commitment in human neural progenitor cells.PLoS Comput Biol. 2015 Mar 20;11(3):e1004106. doi: 10.1371/journal.pcbi.1004106. eCollection 2015 Mar. PLoS Comput Biol. 2015. PMID: 25793621 Free PMC article.
-
A method for spatially resolved local intracellular mechanochemical sensing and organelle manipulation.Biophys J. 2012 Aug 8;103(3):395-404. doi: 10.1016/j.bpj.2012.06.010. Biophys J. 2012. PMID: 22947855 Free PMC article.
-
Phagosomal transport depends strongly on phagosome size.Sci Rep. 2017 Dec 6;7(1):17068. doi: 10.1038/s41598-017-17183-7. Sci Rep. 2017. PMID: 29213131 Free PMC article.
-
Phagocytosis: Our Current Understanding of a Universal Biological Process.Front Immunol. 2020 Jun 2;11:1066. doi: 10.3389/fimmu.2020.01066. eCollection 2020. Front Immunol. 2020. PMID: 32582172 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

