The Arabidopsis immutans mutation affects plastid differentiation and the morphogenesis of white and green sectors in variegated plants
- PMID: 11553735
- PMCID: PMC117963
- DOI: 10.1104/pp.127.1.67
The Arabidopsis immutans mutation affects plastid differentiation and the morphogenesis of white and green sectors in variegated plants
Abstract
The immutans (im) variegation mutant of Arabidopsis has green and white leaf sectors due to the action of a nuclear recessive gene, IMMUTANS (IM). This gene encodes the IM protein, which is a chloroplast homolog of the mitochondrial alternative oxidase. Because the white sectors of im accumulate the noncolored carotenoid, phytoene, IM likely serves as a redox component in phytoene desaturation. In this paper, we show that IM has a global impact on plant growth and development and is required for the differentiation of multiple plastid types, including chloroplasts, amyloplasts, and etioplasts. IM promoter activity and IM mRNAs are also expressed ubiquitously in Arabidopsis. IM transcript levels correlate with carotenoid accumulation in some, but not all, tissues. This suggests that IM function is not limited to carotenogenesis. Leaf anatomy is radically altered in the green and white sectors of im: Mesophyll cell sizes are dramatically enlarged in the green sectors and palisade cells fail to expand in the white sectors. The green im sectors also have significantly higher than normal rates of O(2) evolution and elevated chlorophyll a/b ratios, typical of those found in "sun" leaves. We conclude that the changes in structure and photosynthetic function of the green leaf sectors are part of an adaptive mechanism that attempts to compensate for a lack of photosynthesis in the white leaf sectors, while maximizing the ability of the plant to avoid photodamage.
Figures





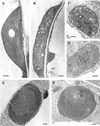


References
-
- Allen JF, Holmes NG. Electron transport and redox titration. In: Hipkins MF, Baker NR, editors. Photosynthesis Energy Transduction: A Practical Approach. Oxford: IRL Press; 1986. pp. 103–140.
-
- Beyer P, Mayer M, Kleinig H. Molecular oxygen and the state of geometric isomerism of intermediates are essential in the carotene desaturation and cyclization reactions in daffodil chromoplasts. Eur J Biochem. 1989;184:141–150. - PubMed
-
- Bowman J. Arabidopsis: An Atlas of Morphology and Development. New York: Springer-Verlag; 1994.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

