Chloroplast biogenesis. Regulation of lipid transport to the thylakoid in chloroplasts isolated from expanding and fully expanded leaves of pea
- PMID: 11553746
- PMCID: PMC117974
- DOI: 10.1104/pp.127.1.184
Chloroplast biogenesis. Regulation of lipid transport to the thylakoid in chloroplasts isolated from expanding and fully expanded leaves of pea
Abstract
To study the regulation of lipid transport from the chloroplast envelope to the thylakoid, intact chloroplasts, isolated from fully expanded or still-expanding pea (Pisum sativum) leaves, were incubated with radiolabeled lipid precursors and thylakoid membranes subsequently were isolated. Incubation with UDP[(3)H]Gal labeled monogalactosyldiacylglycerol in both envelope membranes and digalactosyldiacylglycerol in the outer chloroplast envelope. Galactolipid synthesis increased with incubation temperature. Transport to the thylakoid was slow below 12 degrees C, and exhibited a temperature dependency closely resembling that for the previously reported appearance and disappearance of vesicles in the stroma (D.J. Morré, G. Selldén, C. Sundqvist, A.S. Sandelius [1991] Plant Physiol 97: 1558-1564). In mature chloroplasts, monogalactosyldiacylglycerol transport to the thylakoid was up to three times higher than digalactosyldiacylglycerol transport, whereas the difference was markedly lower in developing chloroplasts. Incubation of chloroplasts with [(14)C]acyl-coenzyme A labeled phosphatidylcholine (PC) and free fatty acids in the inner envelope membrane and phosphatidylglycerol at the chloroplast surface. PC and phosphatidylglycerol were preferentially transported to the thylakoid. Analysis of lipid composition revealed that the thylakoid contained approximately 20% of the chloroplast PC. Our results demonstrate that lipids synthesized at the chloroplast surface as well as in the inner envelope membrane are transported to the thylakoid and that lipid sorting is involved in the process. Furthermore, the results also indicate that more than one pathway exists for galactolipid transfer from the chloroplast envelope to the thylakoid.
Figures
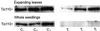
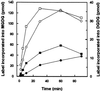




Similar articles
-
(Galacto) lipid export from envelope to thylakoid membranes in intact chloroplasts. II. A general process with a key role for the envelope in the establishment of lipid asymmetry in thylakoid membranes.Biochim Biophys Acta. 1995 Feb 15;1233(2):123-33. doi: 10.1016/0005-2736(94)00248-n. Biochim Biophys Acta. 1995. PMID: 7865537
-
Age-dependent variation in membrane lipid synthesis in leaves of garden pea (Pisum sativum L.).J Exp Bot. 2001 Dec;52(365):2275-82. doi: 10.1093/jexbot/52.365.2275. J Exp Bot. 2001. PMID: 11709577
-
Galactolipid export from envelope to thylakoid membranes in intact chloroplasts. I. Characterization and involvement in thylakoid lipid asymmetry.Biochim Biophys Acta. 1992 Mar 2;1104(2):331-41. doi: 10.1016/0005-2736(92)90048-q. Biochim Biophys Acta. 1992. PMID: 1547267
-
Chloroplast lipid synthesis and lipid trafficking through ER-plastid membrane contact sites.Biochem Soc Trans. 2012 Apr;40(2):457-63. doi: 10.1042/BST20110752. Biochem Soc Trans. 2012. PMID: 22435830 Review.
-
Role of membrane glycerolipids in photosynthesis, thylakoid biogenesis and chloroplast development.J Plant Res. 2016 Jul;129(4):565-580. doi: 10.1007/s10265-016-0827-y. Epub 2016 Apr 25. J Plant Res. 2016. PMID: 27114097 Free PMC article. Review.
Cited by
-
ER-plastid contact sites as molecular crossroads for plastid lipid biosynthesis.BMC Biol. 2025 May 22;23(1):139. doi: 10.1186/s12915-025-02239-2. BMC Biol. 2025. PMID: 40405194 Free PMC article. Review.
-
Chloroplast vesicle transport.Photosynth Res. 2018 Dec;138(3):361-371. doi: 10.1007/s11120-018-0566-0. Epub 2018 Aug 16. Photosynth Res. 2018. PMID: 30117121 Free PMC article. Review.
-
Bioinformatic indications that COPI- and clathrin-based transport systems are not present in chloroplasts: an Arabidopsis model.PLoS One. 2014 Aug 19;9(8):e104423. doi: 10.1371/journal.pone.0104423. eCollection 2014. PLoS One. 2014. PMID: 25137124 Free PMC article.
-
Genes co-expressed with CPSAR1 identified using ATTED-II.Plant Signal Behav. 2010 Sep;5(9):1141-3. doi: 10.4161/psb.5.9.12674. Epub 2010 Sep 1. Plant Signal Behav. 2010. PMID: 20729628 Free PMC article.
-
The K-segment of maize DHN1 mediates binding to anionic phospholipid vesicles and concomitant structural changes.Plant Physiol. 2009 Jul;150(3):1503-14. doi: 10.1104/pp.109.136697. Epub 2009 May 13. Plant Physiol. 2009. PMID: 19439573 Free PMC article.
References
-
- Allan D, Kallen KJ. Transport of lipids to the plasma membrane in animal cells. Prog Lipid Res. 1993;32:195–219. - PubMed
-
- Bahl J, Francke B, Monéger Lipid composition of envelopes, prolamellar bodies and other plastid membranes in etioloated, green and greening wheat leaves. Planta. 1976;129:193–201. - PubMed
-
- Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of Golgi measured by the coupled incorporation of N-acetyl glucosamine. Cell. 1984;39:405–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

