Long-distance phloem transport of glucosinolates in Arabidopsis
- PMID: 11553747
- PMCID: PMC117975
- DOI: 10.1104/pp.127.1.194
Long-distance phloem transport of glucosinolates in Arabidopsis
Abstract
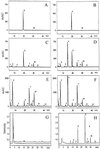
Glucosinolates are a large group of plant secondary metabolites found mainly in the order Capparales, which includes a large number of economically important Brassica crops and the model plant Arabidopsis. In the present study, several lines of evidence are provided for phloem transport of glucosinolates in Arabidopsis. When radiolabeled p-hydroxybenzylglucosinolate (p-OHBG) and sucrose were co-applied to the tip of detached leaves, both tracers were collected in the phloem exudates at the petioles. Long-distance transport of [(14)C]p-OHBG was investigated in wild-type and transgenic 35S::CYP79A1 plants, synthesizing high amounts of p-OHBG, which is not a natural constituent of wild-type Arabidopsis. In both wild-type and 35S::CYP79A1 plants, radiolabeled p-OHBG was rapidly transported from the application site into the whole plant and intact p-OHBG was recovered from different tissues. The pattern of distribution of the radioactivity corresponded to that expected for transport of photoassimilates such as sucrose, and was consistent with translocation in phloem following the source-sink relationship. Radiolabeled p-OHBG was shown to accumulate in the seeds of wild-type and 35S::CYP79A1 plants, where p-OHBG had been either exogenously applied or endogenously synthesized from Tyr in the leaves. p-OHBG was found in phloem exudates collected from cut petioles of leaves from both wild-type and 35S::CYP79A1 plants. Phloem exudates were shown to contain intact glucosinolates, and not desulphoglucosinolates, as the transport form. It is concluded that intact glucosinolates are readily loaded into and transported by the phloem.
Figures


References
-
- Andreasson E. Structural and functional studies of the myrosinase-glucosinolate system in Arabidopsis thaliana and Brassica napus. PhD thesis. Uppsala: Swedish University of Agricultral Sciences; 2000.
-
- Bak S, Olsen CE, Petersen BL, Møller BL, Halkier BA. Metabolic engineering of p-hydroxybenzylglucosinolate in Arabidopsis by expression of the cyanogenic CYP79A1 from Sorghum bicolor. Plant J. 1999;20:663–672. - PubMed
-
- Bartlet E, Kiddle G, Williams I, Wallsgrove RM. Wound-induced increases in the glucosinolate content of oilseed rape and their effect on subsequent herbivory by a crucifer specialist. Entomol Exp Appl. 1999;91:163–167.
-
- Brudenell AJP, Griffiths H, Rossiter JT, Baker DA. The phloem mobility of glucosinolates. J Exp Bot. 1999;50:745–756.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

