N-Acylphosphatidylethanolamine accumulation in potato cells upon energy shortage caused by anoxia or respiratory inhibitors
- PMID: 11553752
- PMCID: PMC117980
- DOI: 10.1104/pp.127.1.240
N-Acylphosphatidylethanolamine accumulation in potato cells upon energy shortage caused by anoxia or respiratory inhibitors
Abstract
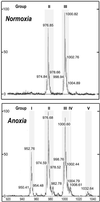
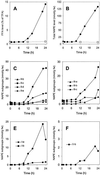
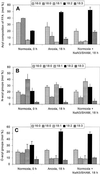
A minor phospholipid was isolated from potato (Solanum tuberosum L. cv Bintje) cells, chromatographically purified, and identified by electrospray ionization mass spectrometry as N-acylphosphatidylethanolamine (NAPE). The NAPE level was low in unstressed cells (13 +/- 4 nmol g fresh weight(-1)). According to acyl chain length, only 16/18/18 species (group II) and 18/18/18 species (group III) were present. NAPE increased up to 13-fold in anoxia-stressed cells, but only when free fatty acids (FFAs) started being released, after about 10 h of treatment. The level of groups II and III was increased by unspecific N-acylation of phosphatidylethanolamine, and new 16/16/18 species (group I) appeared via N-palmitoylation. NAPE also accumulated in aerated cells treated with NaN(3) plus salicylhydroxamate. N-acyl patterns of NAPE were dominated by 18:1, 18:2, and 16:0, but never reflected the FFA composition. Moreover, they did not change greatly after the treatments, in contrast with O-acyl patterns. Anoxia-induced NAPE accumulation is rooted in the metabolic homeostasis failure due to energy deprivation, but not in the absence of O(2), and is part of an oncotic death process. The acyl composition of basal and stress-induced NAPE suggests the existence of spatially distinct FFA and phosphatidylethanolamine pools. It reflects the specificity of NAPE synthase, the acyl composition, localization and availability of substrates, which are intrinsic cell properties, but has no predictive value as to the type of stress imposed. Whether NAPE has a physiological role depends on the cell being still alive and its compartmentation maintained during the stress period.
Figures


References
-
- Akoka S, Tellier C, LeRoux C, Marion D. A phosphorous magnetic resonance spectroscopy and a differential calorimetry study of the physical properties of N-acylphosphatidylethanolamines in aqueous dispersions. Chem Phys Lipids. 1988;46:43–50.
-
- Browse J, Somerville C. Glycerolipid synthesis: biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:467–506.
-
- Chapman KD. Emerging physiological roles for N-acylphosphatidylethanolamine metabolism in plants: signal transduction and membrane protection. Chem Phys Lipids. 2000;108:221–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

