Characterization of two HKT1 homologues from Eucalyptus camaldulensis that display intrinsic osmosensing capability
- PMID: 11553756
- PMCID: PMC117984
- DOI: 10.1104/pp.127.1.283
Characterization of two HKT1 homologues from Eucalyptus camaldulensis that display intrinsic osmosensing capability
Abstract
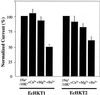
Plants have multiple potassium (K(+)) uptake and efflux mechanisms that are expressed throughout plant tissues to fulfill different physiological functions. Several different classes of K(+) channels and carriers have been identified at the molecular level in plants. K(+) transporters of the HKT1 superfamily have been cloned from wheat (Triticum aestivum), Arabidopsis, and Eucalyptus camaldulensis. The functional characteristics as well as the primary structure of these transporters are diverse with orthologues found in bacterial and fungal genomes. In this report, we provide a detailed characterization of the functional characteristics, as expressed in Xenopus laevis oocytes, of two cDNAs isolated from E. camaldulensis that encode proteins belonging to the HKT1 superfamily of K(+)/Na(+) transporters. The transport of K(+) in EcHKT-expressing oocytes is enhanced by Na(+), but K(+) was also transported in the absence of Na(+). Na(+) is transported in the absence of K(+) as has been demonstrated for HKT1 and AtHKT1. Overall, the E. camaldulensis transporters show some similarities and differences in ionic selectivity to HKT1 and AtHKT1. One striking difference between HKT1 and EcHKT is the sensitivity to changes in the external osmolarity of the solution. Hypotonic solutions increased EcHKT induced currents in oocytes by 100% as compared with no increased current in HKT1 expressing or uninjected oocytes. These osmotically sensitive currents were not enhanced by voltage and may mediate water flux. The physiological function of these osmotically induced increases in currents may be related to the ecological niches that E. camaldulensis inhabits, which are periodically flooded. Therefore, the osmosensing function of EcHKT may provide this species with a competitive advantage in maintaining K(+) homeostasis under certain conditions.
Figures



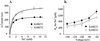



Similar articles
-
Characterisation of two distinct HKT1-like potassium transporters from Eucalyptus camaldulensis.Plant Mol Biol. 2000 Jul;43(4):515-25. doi: 10.1023/a:1006496402463. Plant Mol Biol. 2000. PMID: 11052203
-
The Arabidopsis HKT1 gene homolog mediates inward Na(+) currents in xenopus laevis oocytes and Na(+) uptake in Saccharomyces cerevisiae.Plant Physiol. 2000 Apr;122(4):1249-59. doi: 10.1104/pp.122.4.1249. Plant Physiol. 2000. PMID: 10759522 Free PMC article.
-
Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants.Proc Natl Acad Sci U S A. 2002 Apr 30;99(9):6428-33. doi: 10.1073/pnas.082123799. Epub 2002 Apr 16. Proc Natl Acad Sci U S A. 2002. PMID: 11959905 Free PMC article.
-
To exclude or to accumulate? Revealing the role of the sodium HKT1;5 transporter in plant adaptive responses to varying soil salinity.Plant Physiol Biochem. 2021 Dec;169:333-342. doi: 10.1016/j.plaphy.2021.11.030. Epub 2021 Nov 19. Plant Physiol Biochem. 2021. PMID: 34837866 Review.
-
Role and Functional Differences of HKT1-Type Transporters in Plants under Salt Stress.Int J Mol Sci. 2019 Mar 1;20(5):1059. doi: 10.3390/ijms20051059. Int J Mol Sci. 2019. PMID: 30823627 Free PMC article. Review.
Cited by
-
Expression of the cation transporter McHKT1 in a halophyte.Plant Mol Biol. 2003 Jul;52(5):967-80. doi: 10.1023/a:1025445612244. Plant Mol Biol. 2003. PMID: 14558658
-
Salt stress affects xylem differentiation of grey poplar (Populus x canescens).Planta. 2009 Jan;229(2):299-309. doi: 10.1007/s00425-008-0829-7. Epub 2008 Oct 23. Planta. 2009. PMID: 18946679
-
Structural variations in wheat HKT1;5 underpin differences in Na+ transport capacity.Cell Mol Life Sci. 2018 Mar;75(6):1133-1144. doi: 10.1007/s00018-017-2716-5. Epub 2017 Nov 27. Cell Mol Life Sci. 2018. PMID: 29177534 Free PMC article.
-
Cell signaling during cold, drought, and salt stress.Plant Cell. 2002;14 Suppl(Suppl):S165-83. doi: 10.1105/tpc.000596. Plant Cell. 2002. PMID: 12045276 Free PMC article. Review. No abstract available.
-
Heterelogous expression of plant genes.Int J Plant Genomics. 2009;2009:296482. doi: 10.1155/2009/296482. Epub 2009 Aug 6. Int J Plant Genomics. 2009. PMID: 19672459 Free PMC article.
References
-
- Amtmann A, Sanders D. Mechanisms of Na+ uptake by plant cells. Adv Bot Res. 1999;29:75–112.
-
- Arabidopsis Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis. Nature. 2000;408:796–815. - PubMed
-
- Bihler H, Gaber RF, Slayman CL, Bertl A. The presumed potassium carrier Trk2p in Saccharomyces cerevisiae determines an H+-dependent, K+-independent current. FEBS Lett. 1999;447:115–120. - PubMed
-
- Blount P, Moe PC. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol. 1999;7:420–424. - PubMed
-
- Box S, Schachtman DP. The effect of low concentrations of sodium on potassium uptake and growth of wheat. Aust J Plant Physiol. 2000;27:175–182.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

