RL-37, an alpha-helical antimicrobial peptide of the rhesus monkey
- PMID: 11557457
- PMCID: PMC90719
- DOI: 10.1128/AAC.45.10.2695-2702.2001
RL-37, an alpha-helical antimicrobial peptide of the rhesus monkey
Abstract
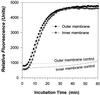
Rhesus monkey bone marrow expresses a cathelicidin whose C-terminal domain comprises a 37-residue alpha-helical peptide (RL-37) that resembles human LL-37. Like its human counterpart, RL-37 rapidly permeabilized the membranes of Escherichia coli ML-35p and lysed liposomes that simulated bacterial membranes. When tested in media whose NaCl concentrations approximated those of extracellular fluids, RL-37 was considerably more active than LL-37 against staphylococci. Whereas human LL-37 contains five acidic residues and has a net charge of +6, rhesus RL-37 has only two acidic residues and a net charge of +8. Speculating that the multiple acidic residues of human LL-37 reduced its efficacy against staphylococci, we made a peptide (LL-37 pentamide) in which each aspartic acid of LL-37 was replaced by an asparagine and each glutamic acid was replaced by a glutamine. LL-37 pentamide's antistaphylococcal activity was substantially greater than that of LL-37. Thus, although the precursor of LL-37 is induced in human skin keratinocytes by injury or inflammation, its insufficiently cationic antimicrobial domain may contribute to the success of staphylococci in colonizing and infecting human skin.
Figures






Similar articles
-
Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37.Antimicrob Agents Chemother. 2005 Jul;49(7):2845-50. doi: 10.1128/AAC.49.7.2845-2850.2005. Antimicrob Agents Chemother. 2005. PMID: 15980359 Free PMC article.
-
In silico identification and biological evaluation of antimicrobial peptides based on human cathelicidin LL-37.Antimicrob Agents Chemother. 2006 Sep;50(9):2983-9. doi: 10.1128/AAC.01583-05. Antimicrob Agents Chemother. 2006. PMID: 16940092 Free PMC article.
-
The C-Terminal VPRTES Tail of LL-37 Influences the Mode of Attachment to a Lipid Bilayer and Antimicrobial Activity.Biochemistry. 2019 May 14;58(19):2447-2462. doi: 10.1021/acs.biochem.8b01297. Epub 2019 May 1. Biochemistry. 2019. PMID: 31016971
-
Structure and biology of cathelicidins.Adv Exp Med Biol. 2000;479:203-18. doi: 10.1007/0-306-46831-X_17. Adv Exp Med Biol. 2000. PMID: 10897421 Review. No abstract available.
-
Human antimicrobial peptides: defensins, cathelicidins and histatins.Biotechnol Lett. 2005 Sep;27(18):1337-47. doi: 10.1007/s10529-005-0936-5. Biotechnol Lett. 2005. PMID: 16215847 Review.
Cited by
-
A member of the cathelicidin family of antimicrobial peptides is produced in the upper airway of the chinchilla and its mRNA expression is altered by common viral and bacterial co-pathogens of otitis media.Mol Immunol. 2007 Mar;44(9):2446-58. doi: 10.1016/j.molimm.2006.10.008. Epub 2006 Nov 20. Mol Immunol. 2007. PMID: 17113647 Free PMC article.
-
Wall teichoic acid deficiency in Staphylococcus aureus confers selective resistance to mammalian group IIA phospholipase A(2) and human beta-defensin 3.Infect Immun. 2008 May;76(5):2169-76. doi: 10.1128/IAI.01705-07. Epub 2008 Mar 17. Infect Immun. 2008. PMID: 18347049 Free PMC article.
-
Proteomic analysis of cervical-vaginal fluid: identification of novel biomarkers for detection of intra-amniotic infection.J Proteome Res. 2007 Jan;6(1):89-96. doi: 10.1021/pr060149v. J Proteome Res. 2007. PMID: 17203952 Free PMC article.
-
In Vitro, In Vivo and In Silico Assessment of the Antimicrobial and Immunomodulatory Effects of a Water Buffalo Cathelicidin (WBCATH) in Experimental Pulmonary Tuberculosis.Antibiotics (Basel). 2022 Dec 31;12(1):75. doi: 10.3390/antibiotics12010075. Antibiotics (Basel). 2022. PMID: 36671276 Free PMC article.
-
Antimicrobial peptides: current status and therapeutic potential.Drugs. 2003;63(4):389-406. doi: 10.2165/00003495-200363040-00005. Drugs. 2003. PMID: 12558461 Review.
References
-
- Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, Gudmundsson G H. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. - PubMed
-
- Agerberth B, Lee J Y, Bergman T, Carlquist M, Boman H G, Mutt V, Jornvall H. Amino acid sequence of PR-39. Isolation from pig intestine of a new member of the family of proline-arginine-rich antibacterial peptides. Eur J Biochem. 1991;202:849–854. - PubMed
-
- Bagella L, Scocchi M, Zanetti M. cDNA sequences of three sheep myeloid cathelicidins. FEBS Lett. 1995;376:225–228. - PubMed
-
- Bruch M D, Dhingra M M, Gierasch L M. Side chain-backbone hydrogen bonding contributes to helix stability in peptides derived from an alpha-helical region of carboxypeptidase A. Proteins. 1991;10:130–139. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

