Inhibition of trypsin-like cysteine proteinases (gingipains) from Porphyromonas gingivalis by tetracycline and its analogues
- PMID: 11557483
- PMCID: PMC90745
- DOI: 10.1128/AAC.45.10.2871-2876.2001
Inhibition of trypsin-like cysteine proteinases (gingipains) from Porphyromonas gingivalis by tetracycline and its analogues
Abstract
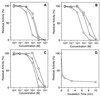
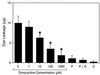
Extracellular cysteine proteinases, referred to as gingipains, are considered important virulence factors for Porphyromonas gingivalis, a bacterium recognized as a major etiologic agent of chronic periodontitis. We investigated the effect of tetracycline and its analogues, doxycycline and minocycline, on the enzymatic activities of gingipains. Tetracyclines at 100 microM totally inhibited the amidolytic activity of arginine-specific gingipains (HRgpA and RgpB). In contrast, inhibition of Kgp was less efficient and required a somewhat higher concentration of the antibiotic to achieve the same effect. Among tetracycline derivatives, the most potent gingipain inhibitor was doxycycline, followed by tetracycline and minocycline. RgpB was inhibited by doxycycline in an uncompetitive and reversible manner with a 50% inhibitory concentration of 3 microM. Significantly, inhibition was unaffected by calcium, excluding the chelating activity of tetracyclines as the mechanism of gingipain inactivation. In contrast, the inhibitory activities of the tetracyclines were reduced by cysteine, a reducing agent, suggesting an interference of the drug at the oxidative region with the catalytic system of the enzyme. Doxycycline, at 10 microM, significantly inhibited the RgpB-mediated production of vascular permeability-enhancing activity from human plasma, thus proving an effective inhibition of gingipain in vivo. These results indicate a new activity of tetracyclines as cysteine proteinase inhibitors and may explain the therapeutic efficiency of these antibiotics in the treatment of periodontitis.
Figures


Similar articles
-
Inhibition of proteolytic, serpinolytic, and progelatinase-b activation activities of periodontopathogens by doxycycline and the non-antimicrobial chemically modified tetracycline derivatives.J Periodontol. 2002 Jan;73(1):79-85. doi: 10.1902/jop.2002.73.1.79. J Periodontol. 2002. PMID: 11846203
-
The role of gingipains in the pathogenesis of periodontal disease.J Periodontol. 2003 Jan;74(1):111-8. doi: 10.1902/jop.2003.74.1.111. J Periodontol. 2003. PMID: 12593605 Review.
-
Inhibition of Porphyromonas gingivalis proteinases (gingipains) by chlorhexidine: synergistic effect of Zn(II).Oral Microbiol Immunol. 2006 Aug;21(4):212-7. doi: 10.1111/j.1399-302X.2006.00277.x. Oral Microbiol Immunol. 2006. PMID: 16842504
-
Proteolysis of human monocyte CD14 by cysteine proteinases (gingipains) from Porphyromonas gingivalis leading to lipopolysaccharide hyporesponsiveness.J Immunol. 2000 Jul 1;165(1):411-8. doi: 10.4049/jimmunol.165.1.411. J Immunol. 2000. PMID: 10861079
-
Suppression of virulence of Porphyromonas gingivalis by potent inhibitors specific for gingipains.Curr Protein Pept Sci. 2003 Dec;4(6):451-8. doi: 10.2174/1389203033486992. Curr Protein Pept Sci. 2003. PMID: 14683430 Review.
Cited by
-
Strategies for the inhibition of gingipains for the potential treatment of periodontitis and associated systemic diseases.J Oral Microbiol. 2014 Aug 18;6. doi: 10.3402/jom.v6.24800. eCollection 2014. J Oral Microbiol. 2014. PMID: 25206939 Free PMC article. Review.
-
The RgpB C-terminal domain has a role in attachment of RgpB to the outer membrane and belongs to a novel C-terminal-domain family found in Porphyromonas gingivalis.J Bacteriol. 2006 Sep;188(17):6376-86. doi: 10.1128/JB.00731-06. J Bacteriol. 2006. PMID: 16923905 Free PMC article.
-
Local Drug Delivery Systems as Novel Approach for Controlling NETosis in Periodontitis.Pharmaceutics. 2024 Sep 6;16(9):1175. doi: 10.3390/pharmaceutics16091175. Pharmaceutics. 2024. PMID: 39339210 Free PMC article. Review.
-
Profiling Protein Arginine Deiminase 4 (PAD4): a novel screen to identify PAD4 inhibitors.Bioorg Med Chem. 2008 Jan 15;16(2):739-45. doi: 10.1016/j.bmc.2007.10.021. Epub 2007 Oct 13. Bioorg Med Chem. 2008. PMID: 17964793 Free PMC article.
-
Oxytetracycline versus Doxycycline Collagen Sponges Designed as Potential Carrier Supports in Biomedical Applications.Pharmaceutics. 2019 Jul 24;11(8):363. doi: 10.3390/pharmaceutics11080363. Pharmaceutics. 2019. PMID: 31344927 Free PMC article.
References
-
- Atilla A, Balcan M, Biçakçi N, Kazandi A. The effect of non-surgical periodontal and adjunctive minocycline-HCl treatments on the activity of salivary proteases. J Periodontol. 1996;67:1–6. - PubMed
-
- Bar-Shavit R, Kahn A, Fenton II J W, Wilner G D. Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science. 1983;220:728–730. - PubMed
-
- Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–484. - PubMed
-
- Campistron G, Coulais Y, Caillard C, Mosser J, Pontagnier H, Houin G. Pharmacokinetics and bioavailability of doxycycline in humans. Arzneim Forsch. 1986;36:1705–1707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

