Homing endonucleases: structural and functional insight into the catalysts of intron/intein mobility
- PMID: 11557808
- PMCID: PMC55915
- DOI: 10.1093/nar/29.18.3757
Homing endonucleases: structural and functional insight into the catalysts of intron/intein mobility
Abstract
Homing endonucleases confer mobility to their host intervening sequence, either an intron or intein, by catalyzing a highly specific double-strand break in a cognate allele lacking the intervening sequence. These proteins are characterized by their ability to bind long DNA target sites (14-40 bp) and their tolerance of minor sequence changes in these sites. A wealth of biochemical and structural data has been generated for these enzymes over the past few years. Herein we review our current understanding of homing endonucleases, including their diversity and evolution, DNA-binding and catalytic mechanisms, and attempts to engineer them to bind novel DNA substrates.
Figures
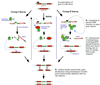
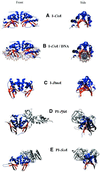

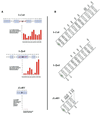
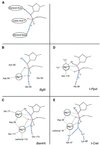

References
-
- Dujon B., Belfort,M., Butow,R.A., Jacq,C., Lemieux,C., Perlman,P.S. and Vogt,V.M. (1989) Mobile introns: definition of terms and recommended nomenclature. Gene, 82, 115–118. - PubMed
-
- Coen D., Deutsch,J., Netter,P., Petrochilo,E. and Slonimski,P.P. (1970) Mitochondrial genetics. I. Methodology and phenomenology. Symp. Soc. Exp. Biol., 23, 449–496. - PubMed
-
- Dujon B. (1980) Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell, 20, 185–197. - PubMed
-
- Bos J.L., Heyting,C., Borst,P., Arnberg,A.C. and Van Bruggen,E.F. (1978) An insert in the single gene for the large ribosomal RNA in yeast mitochondrial DNA. Nature, 275, 336–338. - PubMed
-
- Colleaux L., d’Auriol,L., Betermier,M., Cottarel,G., Jacquier,A., Galibert,F. and Dujon,B. (1986) Universal code equivalent of a yeast mitochondrial intron reading frame is expressed into E.coli as a specific double strand endonuclease. Cell, 44, 521–533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

